The fslE homolog, FTL_0439 (fupA/B), mediates siderophore-dependent iron uptake in Francisella tularensis LVS
- PMID: 20696823
- PMCID: PMC2950358
- DOI: 10.1128/IAI.00503-10
The fslE homolog, FTL_0439 (fupA/B), mediates siderophore-dependent iron uptake in Francisella tularensis LVS
Abstract
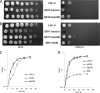
The Gram-negative pathogen Francisella tularensis secretes a siderophore to obtain essential iron by a TonB-independent mechanism. The fslABCDE locus, encoding siderophore-related functions, is conserved among different Francisella strains. In the virulent strain Schu S4, fslE is essential for siderophore utilization and for growth under conditions of iron limitation. In contrast, we found that deletion of fslE did not affect siderophore utilization by the attenuated live vaccine strain (LVS). We found that one of the fslE paralogs encoded in the LVS genome, FTL_0439 (fupA/B), was able to partially complement a Schu S4 ΔfslE mutant for siderophore utilization. We generated a deletion of fupA/B in LVS and in the LVS ΔfslE background. The ΔfupA/B mutant showed reduced growth under conditions of iron limitation. It was able to secrete but was unable to utilize siderophore. Mutation of both fupA/B and fslE resulted in a growth defect of greater severity. The ΔfupA/B mutants showed a replication defect in J774.1A cells and decreased virulence following intraperitoneal infection in mice. Complementation of the ΔfupA/B mutation in cis restored the ability to utilize siderophore and concomitantly restored virulence. Our results indicate that fupA/B plays a significant role in the siderophore-mediated iron uptake mechanism of LVS whereas fslE appears to play a secondary role. Variation in iron acquisition mechanisms may contribute to virulence differences between the strains.
Figures





References
-
- Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. - PubMed
-
- Eigelsbach, H. T., and C. M. Downs. 1961. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J. Immunol. 87:415-425. - PubMed
-
- Elkins, K. L., S. C. Cowley, and C. M. Bosio. 2003. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 5:135-142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

