Survival defects of Cryptococcus neoformans mutants exposed to human cerebrospinal fluid result in attenuated virulence in an experimental model of meningitis
- PMID: 20696827
- PMCID: PMC2950369
- DOI: 10.1128/IAI.00551-10
Survival defects of Cryptococcus neoformans mutants exposed to human cerebrospinal fluid result in attenuated virulence in an experimental model of meningitis
Abstract
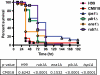
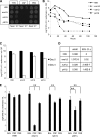
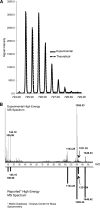
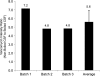
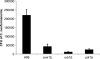
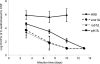
Cryptococcus neoformans is a fungal pathogen that encounters various microenvironments during growth in the mammalian host, including intracellular vacuoles, blood, and cerebrospinal fluid (CSF). Because the CSF is isolated by the blood-brain barrier, we hypothesize that CSF presents unique stresses that C. neoformans must overcome to establish an infection. We assayed 1,201 mutants for survival defects in growth media, saline, and human CSF. We assessed CSF-specific mutants for (i) mutant survival in both human bronchoalveolar lavage (BAL) fluid and fetal bovine serum (FBS), (ii) survival in macrophages, and (iii) virulence using both Caenorhabditis elegans and rabbit models of cryptococcosis. Thirteen mutants exhibited significant survival defects unique to CSF. The mutations of three of these mutants were recreated in the clinical serotype A strain H99: deletions of the genes for a cation ATPase transporter (ena1Δ), a putative NEDD8 ubiquitin-like protein (rub1Δ), and a phosphatidylinositol 4-kinase (pik1Δ). Mutant survival rates in yeast media, saline, and BAL fluid were similar to those of the wild type; however, survival in FBS was reduced but not to the levels in CSF. These mutant strains also exhibited decreased intracellular survival in macrophages, various degrees of virulence in nematodes, and severe attenuation of survival in a rabbit meningitis model. We analyzed the CSF by mass spectrometry for candidate compounds responsible for the survival defect. Our findings indicate that the genes required for C. neoformans survival in CSF ex vivo are necessary for survival and infection in this unique host environment.
Figures



References
-
- Ackermann, K., A. Waxmann, C. V. Glover, and W. Pyerin. 2001. Genes targeted by protein kinase CK2: a genome-wide expression array analysis in yeast. Mol. Cell. Biochem. 227:59-66. - PubMed
-
- Ahmed, K., D. A. Gerber, and C. Cochet. 2002. Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol. 12:226-230. - PubMed
-
- Appelbaum, P. C. 2006. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 12(Suppl. 1):16-23. - PubMed
-
- Bairamian, D., C. E. Johanson, J. T. Parmelee, and M. H. Epstein. 1991. Potassium co-transport with sodium and chloride in the choroid plexus. J. Neurochem. 56:1623-1629. - PubMed
-
- Bañuelos, M. A., and A. Rodríguez-Navarro. 1998. P-type ATPases mediate sodium and potassium effluxes in Schwanniomyces occidentalis. J. Biol. Chem. 273:1640-1646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

