Platelet activation and biofilm formation by Aerococcus urinae, an endocarditis-causing pathogen
- PMID: 20696834
- PMCID: PMC2950351
- DOI: 10.1128/IAI.00469-10
Platelet activation and biofilm formation by Aerococcus urinae, an endocarditis-causing pathogen
Abstract
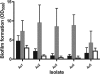
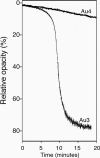
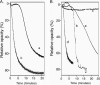
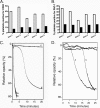
The Gram-positive bacterium Aerococcus urinae can cause infectious endocarditis (IE) in older persons. Biofilm formation and platelet aggregation are believed to contribute to bacterial virulence in IE. Five A. urinae isolates from human blood were shown to form biofilms in vitro, and biofilm formation was enhanced by the presence of human plasma. Four of the A. urinae isolates caused platelet aggregation in platelet-rich plasma from healthy donors. The Au3 isolate, which induced platelet aggregation in all donors, also activated platelets, as determined by flow cytometry. Platelet aggregation was dependent on bacterial protein structures and on platelet activation since it was sensitive to both trypsin and prostaglandin E(1). Plasma proteins at the bacterial surface were needed for platelet aggregation; and roles of the complement system, fibrinogen, and immunoglobulin G were demonstrated. Complement-depleted serum was unable to support platelet aggregation by Au3 and complement blockade using compstatin-inhibited platelet activation. Platelet activation by Au3 was inhibited by blocking of the platelet fibrinogen receptor, and this isolate was also shown to bind to radiolabeled fibrinogen. Removal of IgG from platelet-rich plasma by a specific protease inhibited the platelet aggregation induced by A. urinae, and blockade of the platelet FcRγIIa hindered platelet activation induced by Au3. Convalescent-phase serum from a patient with A. urinae IE transferred the ability of the bacterium to aggregate platelets in an otherwise nonresponsive donor. Our results show that A. urinae exhibits virulence strategies of importance for IE.
Figures


References
-
- Aguirre, M., and M. D. Collins. 1992. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J. Gen. Microbiol. 138:401-405. - PubMed
-
- Astudillo, L., L. Sailler, L. Porte, J. C. Lefevre, P. Massip, and E. Arlet-Suau. 2003. Spondylodiscitis due to Aerococcus urinae: a first report. Scand. J. Infect. Dis. 35:890-891. - PubMed
-
- Bayer, A. S., D. Cheng, M. R. Yeaman, G. R. Corey, R. S. McClelland, L. J. Harrel, and V. G. Fowler, Jr. 1998. In vitro resistance to thrombin-induced platelet microbicidal protein among clinical bacteremic isolates of Staphylococcus aureus correlates with an endovascular infectious source. Antimicrob. Agents Chemother. 42:3169-3172. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

