Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes
- PMID: 20696890
- PMCID: PMC2930590
- DOI: 10.1073/pnas.1005101107
Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes
Abstract
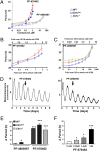
Circadian pacemaking requires the orderly synthesis, posttranslational modification, and degradation of clock proteins. In mammals, mutations in casein kinase 1 (CK1) epsilon or delta can alter the circadian period, but the particular functions of the WT isoforms within the pacemaker remain unclear. We selectively targeted WT CK1epsilon and CK1delta using pharmacological inhibitors (PF-4800567 and PF-670462, respectively) alongside genetic knockout and knockdown to reveal that CK1 activity is essential to molecular pacemaking. Moreover, CK1delta is the principal regulator of the clock period: pharmacological inhibition of CK1delta, but not CK1epsilon, significantly lengthened circadian rhythms in locomotor activity in vivo and molecular oscillations in the suprachiasmatic nucleus (SCN) and peripheral tissue slices in vitro. Period lengthening mediated by CK1delta inhibition was accompanied by nuclear retention of PER2 protein both in vitro and in vivo. Furthermore, phase mapping of the molecular clockwork in vitro showed that PF-670462 treatment lengthened the period in a phase-specific manner, selectively extending the duration of PER2-mediated transcriptional feedback. These findings suggested that CK1delta inhibition might be effective in increasing the amplitude and synchronization of disrupted circadian oscillators. This was tested using arrhythmic SCN slices derived from Vipr2(-/-) mice, in which PF-670462 treatment transiently restored robust circadian rhythms of PER2::Luc bioluminescence. Moreover, in mice rendered behaviorally arrhythmic by the Vipr2(-/-) mutation or by constant light, daily treatment with PF-670462 elicited robust 24-h activity cycles that persisted throughout treatment. Accordingly, selective pharmacological targeting of the endogenous circadian regulator CK1delta offers an avenue for therapeutic modulation of perturbed circadian behavior.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


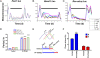

Similar articles
-
The Tau mutation of casein kinase 1ε sets the period of the mammalian pacemaker via regulation of Period1 or Period2 clock proteins.J Biol Rhythms. 2014 Apr;29(2):110-8. doi: 10.1177/0748730414520663. J Biol Rhythms. 2014. PMID: 24682205 Free PMC article.
-
Casein kinase 1 delta regulates the pace of the mammalian circadian clock.Mol Cell Biol. 2009 Jul;29(14):3853-66. doi: 10.1128/MCB.00338-09. Epub 2009 May 4. Mol Cell Biol. 2009. PMID: 19414593 Free PMC article.
-
Selective inhibition of casein kinase 1 epsilon minimally alters circadian clock period.J Pharmacol Exp Ther. 2009 Aug;330(2):430-9. doi: 10.1124/jpet.109.151415. Epub 2009 May 19. J Pharmacol Exp Ther. 2009. PMID: 19458106
-
The biology of the circadian Ck1epsilon tau mutation in mice and Syrian hamsters: a tale of two species.Cold Spring Harb Symp Quant Biol. 2007;72:261-71. doi: 10.1101/sqb.2007.72.073. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18522517 Review.
-
Casein Kinase 1δ Inhibitors as Promising Therapeutic Agents for Neurodegenerative Disorders.Curr Med Chem. 2022;29(27):4698-4737. doi: 10.2174/0929867329666220301115124. Curr Med Chem. 2022. PMID: 35232339 Review.
Cited by
-
In vivo recording of suprachiasmatic nucleus dynamics reveals a dominant role of arginine vasopressin neurons in circadian pacesetting.PLoS Biol. 2023 Aug 29;21(8):e3002281. doi: 10.1371/journal.pbio.3002281. eCollection 2023 Aug. PLoS Biol. 2023. PMID: 37643163 Free PMC article.
-
Casein kinase 1 delta functions at the centrosome to mediate Wnt-3a-dependent neurite outgrowth.J Cell Biol. 2011 Mar 21;192(6):993-1004. doi: 10.1083/jcb.201011111. J Cell Biol. 2011. PMID: 21422228 Free PMC article.
-
House dust mite and Th2 cytokine-mediated epithelial barrier dysfunction attenuation by KL001 in 16-HBE cells.Tissue Barriers. 2024 Jan 2;12(1):2203841. doi: 10.1080/21688370.2023.2203841. Epub 2023 Apr 20. Tissue Barriers. 2024. PMID: 37079442 Free PMC article.
-
Targeting Casein Kinase 1 (CK1) in Hematological Cancers.Int J Mol Sci. 2020 Nov 27;21(23):9026. doi: 10.3390/ijms21239026. Int J Mol Sci. 2020. PMID: 33261128 Free PMC article. Review.
-
Functional analysis of Casein Kinase 1 in a minimal circadian system.PLoS One. 2013 Jul 25;8(7):e70021. doi: 10.1371/journal.pone.0070021. Print 2013. PLoS One. 2013. PMID: 23936135 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- BB/E022553/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_U105170643/MRC_/Medical Research Council/United Kingdom
- EO232231/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- EO225531/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0900414/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

