Carbohydrate-binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects
- PMID: 20696902
- PMCID: PMC2930570
- DOI: 10.1073/pnas.1005732107
Carbohydrate-binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects
Abstract
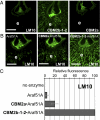
Cell wall degrading enzymes have a complex molecular architecture consisting of catalytic modules and noncatalytic carbohydrate-binding modules (CBMs). The function of CBMs in cell wall degrading processes is poorly understood. Here, we have evaluated the potential enzyme-targeting function of CBMs in the context of intact primary and secondary cell wall deconstruction. The capacity of a pectate lyase to degrade pectic homogalacturonan in primary cell walls was potentiated by cellulose-directed CBMs but not by xylan-directed CBMs. Conversely, the arabinofuranosidase-mediated removal of side chains from arabinoxylan in xylan-rich and cellulose-poor wheat grain endosperm cell walls was enhanced by a xylan-binding CBM but less so by a crystalline cellulose-specific module. The capacity of xylanases to degrade xylan in secondary cell walls was potentiated by both xylan- and cellulose-directed CBMs. These studies demonstrate that CBMs can potentiate the action of a cognate catalytic module toward polysaccharides in intact cell walls through the recognition of nonsubstrate polysaccharides. The targeting actions of CBMs therefore have strong proximity effects within cell wall structures, explaining why cellulose-directed CBMs are appended to many noncellulase cell wall hydrolases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Harris PJ. Diversity in plant cell walls. In: Henry RJ, editor. Plant Diversity and Evolution: Genotypic and Phenotypic Variation in Higher Plants. Wallington, Oxfordshire, UK: CAB International Publishing; 2005. pp. 201–227.
-
- Knox JP. Revealing the structural and functional diversity of plant cell walls. Curr Opin Plant Biol. 2008;11:308–313. - PubMed
-
- Mohnen D. Pectin structure and biosynthesis. Curr Opin Plant Biol. 2008;11:266–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

