Anisotropy of wave propagation in the heart can be modeled by a Riemannian electrophysiological metric
- PMID: 20696934
- PMCID: PMC2930580
- DOI: 10.1073/pnas.1008837107
Anisotropy of wave propagation in the heart can be modeled by a Riemannian electrophysiological metric
Abstract
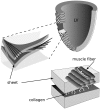
It is well established that wave propagation in the heart is anisotropic and that the ratio of velocities in the three principal directions may be as large as v(f)v(s)v(n) approximately 4(fibers)2(sheets)1(normal). We develop an alternative view of the heart based on this fact by considering it as a non-Euclidean manifold with an electrophysiological(el-) metric based on wave velocity. This metric is more natural than the Euclidean metric for some applications, because el-distances directly encode wave propagation. We develop a model of wave propagation based on this metric; this model ignores higher-order effects like the curvature of wavefronts and the effect of the boundary, but still gives good predictions of local activation times and replicates many of the observed features of isochrones. We characterize this model for the important case of the rotational orthotropic anisotropy seen in cardiac tissue and perform numerical simulations for a slab of cardiac tissue with rotational orthotropic anisotropy and for a model of the ventricles based on diffusion tensor MRI scans of the canine heart. Even though the metric has many slow directions, we show that the rotation of the fibers leads to fast global activation. In the diffusion tensor MRI-based model, with principal velocities 0.25051 m/s, we find examples of wavefronts that eventually reach speeds up to 0.9 m/s and average velocities of 0.7 m/s. We believe that development of this non-Euclidean approach to cardiac anatomy and electrophysiology could become an important tool for the characterization of the normal and abnormal electrophysiological activity of the heart.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Winfree AT, Strogatz SH. Organizing centers for three-dimensional chemical waves. Nature. 1984;311:611–615. - PubMed
-
- Mehra R. Global public health problem of sudden cardiac death. J Electrocardiol. 2007;40:S118–S122. - PubMed
-
- LeGrice IJ, et al. Laminar structure of the heart. II. Mathematical model. Am J Physiol. 1995;269:H571–H582. - PubMed
-
- Caldwell BJ, et al. Three distinct directions of intramural activation reveal nonuniform side-to-side electrical coupling of ventricular myocytes. Circ Arrhythm Electrophysiol. 2009;2(4):433–440. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

