Conformational dynamics of bacteriophage T7 DNA polymerase and its processivity factor, Escherichia coli thioredoxin
- PMID: 20696935
- PMCID: PMC2930546
- DOI: 10.1073/pnas.1010141107
Conformational dynamics of bacteriophage T7 DNA polymerase and its processivity factor, Escherichia coli thioredoxin
Abstract
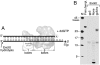
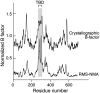
Gene 5 of bacteriophage T7 encodes a DNA polymerase (gp5) responsible for the replication of the phage DNA. Gp5 polymerizes nucleotides with low processivity, dissociating after the incorporation of 1 to 50 nucleotides. Thioredoxin (trx) of Escherichia coli binds tightly (Kd = 5 nM) to a unique segment in the thumb subdomain of gp5 and increases processivity. We have probed the molecular basis for the increase in processivity. A single-molecule experiment reveals differences in rates of enzymatic activity and processivity between gp5 and gp5/trx. Small angle X-ray scattering studies combined with nuclease footprinting reveal two conformations of gp5, one in the free state and one upon binding to trx. Comparative analysis of the DNA binding clefts of DNA polymerases and DNA binding proteins show that the binding surface contains more hydrophobic residues than other DNA binding proteins. The balanced composition between hydrophobic and charged residues of the binding site allows for efficient sliding of gp5/trx on the DNA. We propose a model for trx-induced conformational changes in gp5 that enhance the processivity by increasing the interaction of gp5 with DNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

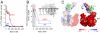

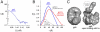
References
-
- Hamdan SM, Richardson CC. Motors, switches, and contacts in the replisome. Annu Rev Biochem. 2009;78:205–243. - PubMed
-
- Huber HE, Russel M, Model P, Richardson CC. Interaction of mutant thioredoxins of Escherichia coli with the gene 5 protein of phage T7: The redox capacity of thioredoxin is not required for stimulation of DNA polymerase activity. J Biol Chem. 1986;261:15006–15012. - PubMed
-
- Hamdan SM, et al. Dynamic DNA helicase-DNA polymerase interactions assure processive replication fork movement. Mol Cell. 2007;27:539–549. - PubMed
-
- Tabor S, Huber HE, Richardson CC. Escherichia coli thioredoxin confers processivity on the DNA polymerase activity of the gene 5 protein of bacteriophage T7. J Biol Chem. 1987;262:16212–16223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

