Amyotrophic lateral sclerosis-frontotemporal lobar dementia in 3 families with p.Ala382Thr TARDBP mutations
- PMID: 20697052
- PMCID: PMC3535689
- DOI: 10.1001/archneurol.2010.173
Amyotrophic lateral sclerosis-frontotemporal lobar dementia in 3 families with p.Ala382Thr TARDBP mutations
Abstract
Background: TAR DNA-binding protein 43, encoded by the TARDBP gene, has been identified as the major pathological protein of frontotemporal lobar dementia (FTLD) with or without amyotrophic lateral sclerosis (ALS) and sporadic ALS. Subsequently, mutations in the TARDBP gene have been detected in 2% to 3% of patients with ALS (both familial and sporadic ALS). However, to our knowledge, there is only 1 description of 2 patients with FTLD and TARDBP gene mutations who later developed motor neuron disease.
Objective: To describe cognitive abnormalities in 3 Italian families with familial ALS and TARDBP gene mutations.
Design, setting, and participants: Genetic, neuropsychological, and neuroimaging analyses in 36 patients with familial non-superoxide dismutase 1 gene (SOD1) ALS and 280 healthy controls. Main Outcome Measure We identified 3 index cases of familial ALS carrying the p.Ala382Thr missense mutation of the TARDBP gene and with clinical, neuroimaging, and neuropsychological features of FTLD.
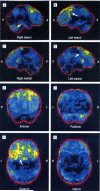
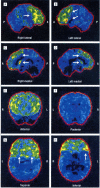
Results: The p.Ala382Thr missense mutation of the TARDBP gene was absent in the 280 controls. It was present in all affected members of the 3 families for whom DNA was available. All affected members of the 3 families developed FTLD after the onset of ALS, confirmed by neuropsychological testing and hypometabolism in frontal associative areas assessed with fludeoxyglucose F 18 positron emission tomography and computed tomography.
Conclusions: Three apparently unrelated families with familial ALS carrying the p.Ala382Thr TARDBP missense mutation developed FTLD. In these families, FTLD cosegregates with ALS. Patients with ALS carrying TARDBP mutations may develop FTLD.
Figures



References
-
- Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):5962. - PubMed
-
- Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):12051208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

