In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study
- PMID: 20697067
- PMCID: PMC2954133
- DOI: 10.1200/JCO.2010.28.9793
In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study
Abstract
Purpose: Combining tumor antigens with an immunostimulant can induce the immune system to specifically eliminate cancer cells. Generally, this combination is accomplished in an ex vivo, customized manner. In a preclinical lymphoma model, intratumoral injection of a Toll-like receptor 9 (TLR9) agonist induced systemic antitumor immunity and cured large, disseminated tumors.
Patients and methods: We treated 15 patients with low-grade B-cell lymphoma using low-dose radiotherapy to a single tumor site and-at that same site-injected the C-G enriched, synthetic oligodeoxynucleotide (also referred to as CpG) TLR9 agonist PF-3512676. Clinical responses were assessed at distant, untreated tumor sites. Immune responses were evaluated by measuring T-cell activation after in vitro restimulation with autologous tumor cells.
Results: This in situ vaccination maneuver was well-tolerated with only grade 1 to 2 local or systemic reactions and no treatment-limiting adverse events. One patient had a complete clinical response, three others had partial responses, and two patients had stable but continually regressing disease for periods significantly longer than that achieved with prior therapies. Vaccination induced tumor-reactive memory CD8 T cells. Some patients' tumors were able to induce a suppressive, regulatory phenotype in autologous T cells in vitro; these patients tended to have a shorter time to disease progression. One clinically responding patient received a second course of vaccination after relapse resulting in a second, more rapid clinical response.
Conclusion: In situ tumor vaccination with a TLR9 agonist induces systemic antilymphoma clinical responses. This maneuver is clinically feasible and does not require the production of a customized vaccine product.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

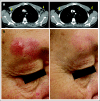
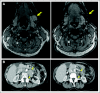
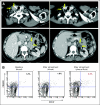
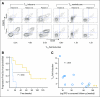
Comment in
-
Harnessing innate immunity to suppress lymphoma.J Clin Oncol. 2010 Oct 1;28(28):4295-6. doi: 10.1200/JCO.2010.30.4212. Epub 2010 Aug 9. J Clin Oncol. 2010. PMID: 20697076 No abstract available.
-
Targeting malignant B cells as antigen-presenting cells: TLR-9 agonist induces systemic regression of lymphoma.Expert Rev Vaccines. 2011 Mar;10(3):295-8. doi: 10.1586/erv.11.6. Expert Rev Vaccines. 2011. PMID: 21434797
References
-
- Rohatiner AZ, Lister TA. The clinical course of follicular lymphoma. Best Pract Res Clin Haematol. 2005;18:1–10. - PubMed
-
- Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. - PubMed
-
- Di Nicola M, Zappasodi R, Carlo-Stella C, et al. Vaccination with autologous tumor-loaded dendritic cells induces clinical and immunologic responses in indolent B-cell lymphoma patients with relapsed and measurable disease: A pilot study. Blood. 2009;113:18–27. - PubMed
-
- Kwak LW, Campbell MJ, Czerwinski DK, et al. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992;327:1209–1215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

