Evolution of CST function in telomere maintenance
- PMID: 20697207
- PMCID: PMC3041159
- DOI: 10.4161/cc.9.16.12547
Evolution of CST function in telomere maintenance
Abstract
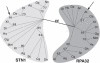
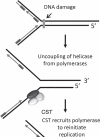
Telomeres consist of an elaborate, higher-order DNA architecture, and a suite of proteins that provide protection for the chromosome terminus by blocking inappropriate recombination and nucleolytic attack. In addition, telomeres facilitate telomeric DNA replication by physical interactions with telomerase and the lagging strand replication machinery. The prevailing view has been that two distinct telomere capping complexes evolved, shelterin in vertebrates and a trimeric complex comprised of Cdc13, Stn1 and Ten1 (CST) in yeast. The recent discovery of a CST-like complex in plants and humans raises new questions about the composition of telomeres and their regulatory mechanisms in multicellular eukaryotes. In this review we discuss the evolving functions and interactions of CST components and their contributions to chromosome end protection and DNA replication.
Figures





References
-
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:18–20. - PubMed
-
- Lei M, Podell ER, Cech TR. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat Struct Mol Biol. 2004;11:1223–1229. - PubMed
-
- Miyoshi T, Kanoh J, Saito M, Ishikawa F. Fission yeast Pot1-Tpp1 protects telomeres and regulates telomere length. Science. 2008;320:1341–1344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
