Substrate-specific modulation of CYP3A4 activity by genetic variants of cytochrome P450 oxidoreductase
- PMID: 20697309
- PMCID: PMC2940949
- DOI: 10.1097/FPC.0b013e32833e0cb5
Substrate-specific modulation of CYP3A4 activity by genetic variants of cytochrome P450 oxidoreductase
Abstract
Objectives: CYP3A4 receives electrons from P450 oxidoreductase (POR) to metabolize about 50% of clinically used drugs. There is substantial inter-individual variation in CYP3A4 catalytic activity that is not explained by CYP3A4 genetic variants. CYP3A4 is flexible and distensible, permitting it to accommodate substrates varying in shape and size. To elucidate the mechanisms of variability in CYP3A4 catalysis, we examined the effects of genetic variants of POR, and explored the possibility that substrate-induced conformational changes in CYP3A4 differentially affect the ability of POR variants to support catalysis.
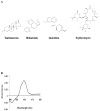
Methods: We expressed human CYP3A4 and four POR variants (Q153R, A287P, R457H, A503 V) in bacteria, reconstituted them in vitro and measured the Michaelis constant and maximum velocity with testosterone, midazolam, quinidine and erythromycin as substrates.
Results: POR A287P and R457H had low activity with all substrates; Q153R had 76-94% of wild-type (WT) activity with midazolam and erythromycin, but 129-150% activity with testosterone and quinidine. The A503 V polymorphism reduced the CYP3A4 activity to 61-77% of WT with testosterone and midazolam, but had nearly WT activity with quinidine and erythromycin.
Conclusion: POR variants affect CYP3A4 activities. The impact of a POR variant on catalysis by CYP3A4 is substrate-specific, probably because of substrate-induced conformational changes in CYP3A4.
Figures

References
-
- Ono T, Bloch K. Solubilization and partial characterization of rat liver squalene epoxidase. J Biol Chem. 1975;250:1571–1579. - PubMed
-
- Nishino H, Ishibashi T. Evidence for requirement of NADPH-cytochrome P450 oxidoreductase in the microsomal NADPH-sterol Delta7-reductase system. Arch Biochem Biophys. 2000;374:293–298. - PubMed
-
- Wilks A, Black SM, Miller WL, Ortiz de Montellano PR. Expression and characterization of truncated human heme oxygenase (hHO-1) and a fusion protein of hHO-1 with human cytochrome P450 reductase. Biochemistry. 1995;34:4421–4427. - PubMed
-
- Vermilion JL, Ballou DP, Massey V, Coon MJ. Separate roles for FMN and FAD in catalysis by liver microsomal NADPH-cytochrome P-450 reductase. J Biol Chem. 1981;256:266–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

