MUC4 mucin-induced epithelial to mesenchymal transition: a novel mechanism for metastasis of human ovarian cancer cells
- PMID: 20697346
- PMCID: PMC3005772
- DOI: 10.1038/onc.2010.309
MUC4 mucin-induced epithelial to mesenchymal transition: a novel mechanism for metastasis of human ovarian cancer cells
Erratum in
- Oncogene. 2010 Nov 11;29(45):6084
Abstract
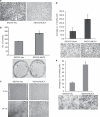
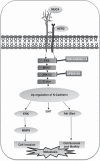
The acquisition of invasiveness in ovarian cancer (OC) is accompanied by the process of epithelial-to-mesenchymal transition (EMT). The MUC4 mucin is overexpressed in ovarian tumors and has a role in the invasiveness of OC cells. The present study was aimed at evaluating the potential involvement of MUC4 in the metastasis of OC cells by inducing EMT. Ectopic overexpression of MUC4 in OC cells (SKOV3-MUC4) resulted in morphological alterations along with a decreased expression of epithelial markers (E-cadherin and cytokeratin (CK)-18) and an increased expression of mesenchymal markers (N-cadherin and vimentin) compared with the control cells (SKOV3-vector). Also, pro-EMT transcription factors TWIST1, TWIST2 and SNAIL showed an upregulation in SKOV3-MUC4 cells. We further investigated the pathways upstream of N-cadherin, such as focal adhesion kinase (FAK), MKK7, JNK1/2 and c-Jun, which were also activated in the SKOV3-MUC4 cells compared with SKOV3-vector cells. Inhibition of phospho-FAK (pFAK) and pJNK1/2 decreased N-cadherin expression in the MUC4-overexpressing cells, which further led to a significant decrease in cellular motility. Knockdown of N-cadherin decreased the activation of extracellular signal-regulated kinase-1/2 (ERK1/2), AKT and matrix metalloproteinase 9 (MMP9), and inhibited the motility in the SKOV3-MUC4 cells. Upon in vivo tumorigenesis and metastasis analysis, the SKOV3-MUC4 cells produced significantly larger tumors and demonstrated a higher incidence of metastasis to distance organs (peritoneal wall, colon, intestine, stomach, lymph nodes, liver and diaphragm). Taken together, our study reveals a novel role for MUC4 in inducing EMT through the upregulation of N-cadherin and promoting metastasis of OC cells.
Figures





Similar articles
-
MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells.Br J Cancer. 2008 Aug 5;99(3):520-6. doi: 10.1038/sj.bjc.6604517. Br J Cancer. 2008. PMID: 18665193 Free PMC article.
-
Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level.Gynecol Oncol. 2005 Apr;97(1):155-65. doi: 10.1016/j.ygyno.2004.12.043. Gynecol Oncol. 2005. PMID: 15790452
-
MUC4 potentiates invasion and metastasis of pancreatic cancer cells through stabilization of fibroblast growth factor receptor 1.Carcinogenesis. 2012 Oct;33(10):1953-64. doi: 10.1093/carcin/bgs225. Epub 2012 Jul 12. Carcinogenesis. 2012. PMID: 22791819 Free PMC article.
-
The epithelial-mesenchymal transition and the estrogen-signaling in ovarian cancer.Curr Drug Targets. 2010 Apr;11(4):474-81. doi: 10.2174/138945010790980385. Curr Drug Targets. 2010. PMID: 20015012 Review.
-
Epithelial-mesenchymal transition in ovarian cancer progression: a crucial role for the endothelin axis.Cells Tissues Organs. 2007;185(1-3):85-94. doi: 10.1159/000101307. Cells Tissues Organs. 2007. PMID: 17587812 Review.
Cited by
-
Extracellular vesicles in ovarian cancer chemoresistance, metastasis, and immune evasion.Cell Death Dis. 2022 Jan 18;13(1):64. doi: 10.1038/s41419-022-04510-8. Cell Death Dis. 2022. PMID: 35042862 Free PMC article. Review.
-
MicroRNA-200c modulates the expression of MUC4 and MUC16 by directly targeting their coding sequences in human pancreatic cancer.PLoS One. 2013 Oct 25;8(10):e73356. doi: 10.1371/journal.pone.0073356. eCollection 2013. PLoS One. 2013. PMID: 24204560 Free PMC article.
-
Methylation-associated silencing of miR-193a-3p promotes ovarian cancer aggressiveness by targeting GRB7 and MAPK/ERK pathways.Theranostics. 2018 Jan 1;8(2):423-436. doi: 10.7150/thno.22377. eCollection 2018. Theranostics. 2018. PMID: 29290818 Free PMC article.
-
Tepotinib Inhibits the Epithelial-Mesenchymal Transition and Tumor Growth of Gastric Cancers by Increasing GSK3β, E-Cadherin, and Mucin 5AC and 6 Levels.Int J Mol Sci. 2020 Aug 21;21(17):6027. doi: 10.3390/ijms21176027. Int J Mol Sci. 2020. PMID: 32825724 Free PMC article.
-
Downregulation of cell surface CA125/MUC16 induces epithelial-to-mesenchymal transition and restores EGFR signalling in NIH:OVCAR3 ovarian carcinoma cells.Br J Cancer. 2011 Mar 15;104(6):989-99. doi: 10.1038/bjc.2011.34. Epub 2011 Feb 15. Br J Cancer. 2011. PMID: 21326240 Free PMC article.
References
-
- Ahmed N, Thompson EW, Quinn MA. Epithelial-mesenchymal interconversions in normal ovarian surface epithelium and ovarian carcinomas: an exception to the norm. J Cell Physiol. 2007;213:581–588. - PubMed
-
- Andrianifahanana M, Moniaux N, Schmied BM, Ringel J, Friess H, Hollingsworth MA, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033–4040. - PubMed
-
- Behrens J. Cadherins as determinants of tissue morphology and suppressors of invasion. Acta Anat (Basel) 1994;149:165–169. - PubMed
-
- Boman F, Buisine MP, Wacrenier A, Querleu D, Aubert JP, Porchet N. Mucin gene transcripts in benign and borderline mucinous tumours of the ovary: an in situ hybridization study. J Pathol. 2001;193:339–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

