Binding of pro-prion to filamin A: by design or an unfortunate blunder
- PMID: 20697352
- PMCID: PMC3159184
- DOI: 10.1038/onc.2010.307
Binding of pro-prion to filamin A: by design or an unfortunate blunder
Abstract
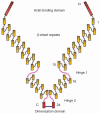
Over the last decades, cancer research has focused on tumor suppressor genes and oncogenes. Genes in other cellular pathways has received less attention. Between 0.5% to 1% of the mammalian genome encodes for proteins that are tethered on the cell membrane via a glycosylphosphatidylinositol (GPI)-anchor. The GPI modification pathway is complex and not completely understood. Prion (PrP), a GPI-anchored protein, is infamous for being the only normal protein that when misfolded can cause and transmit a deadly disease. Though widely expressed and highly conserved, little is known about the functions of PrP. Pancreatic cancer and melanoma cell lines express PrP. However, in these cell lines the PrP exists as a pro-PrP as defined by retaining its GPI anchor peptide signal sequence (GPI-PSS). Unexpectedly, the GPI-PSS of PrP has a filamin A (FLNA) binding motif and binds FLNA. FLNA is a cytolinker protein, and an integrator of cell mechanics and signaling. Binding of pro-PrP to FLNA disrupts the normal FLNA functions. Although normal pancreatic ductal cells lack PrP, about 40% of patients with pancreatic ductal cell adenocarcinoma express PrP in their cancers. These patients have significantly shorter survival time compared with patients whose cancers lack PrP. Pro-PrP is also detected in melanoma in situ but is undetectable in normal melanocyte, and invasive melanoma expresses more pro-PrP. In this review, we will discuss the underlying mechanisms by which binding of pro-PrP to FLNA disrupts normal cellular physiology and contributes to tumorigenesis, and the potential mechanisms that cause the accumulation of pro-PrP in cancer cells.
Figures


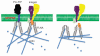
Similar articles
-
Binding of pro-prion to filamin A disrupts cytoskeleton and correlates with poor prognosis in pancreatic cancer.J Clin Invest. 2009 Sep;119(9):2725-36. doi: 10.1172/JCI39542. Epub 2009 Aug 17. J Clin Invest. 2009. PMID: 19690385 Free PMC article.
-
Pro-prion binds filamin A, facilitating its interaction with integrin beta1, and contributes to melanomagenesis.J Biol Chem. 2010 Sep 24;285(39):30328-39. doi: 10.1074/jbc.M110.147413. Epub 2010 Jul 21. J Biol Chem. 2010. PMID: 20650901 Free PMC article.
-
The fatal attraction between pro-prion and filamin A: prion as a marker in human cancers.Biomark Med. 2010 Jun;4(3):453-64. doi: 10.2217/bmm.10.14. Biomark Med. 2010. PMID: 20550479 Free PMC article.
-
Prion Protein Family Contributes to Tumorigenesis via Multiple Pathways.Adv Exp Med Biol. 2017;1018:207-224. doi: 10.1007/978-981-10-5765-6_13. Adv Exp Med Biol. 2017. PMID: 29052140 Review.
-
[Elusive function of prion protein].Nihon Rinsho. 2007 Aug;65(8):1385-90. Nihon Rinsho. 2007. PMID: 17695273 Review. Japanese.
Cited by
-
PRNP is a pan-cancer prognostic and immunity-related to EMT in colorectal cancer.Front Cell Dev Biol. 2024 Aug 5;12:1391873. doi: 10.3389/fcell.2024.1391873. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39170916 Free PMC article.
-
ST8SIA6 Sialylates CD24 to Enhance Its Membrane Localization in BRCA.Cells. 2024 Dec 26;14(1):9. doi: 10.3390/cells14010009. Cells. 2024. PMID: 39791710 Free PMC article.
-
Intracellular CD24 disrupts the ARF-NPM interaction and enables mutational and viral oncogene-mediated p53 inactivation.Nat Commun. 2015 Jan 20;6:5909. doi: 10.1038/ncomms6909. Nat Commun. 2015. PMID: 25600590 Free PMC article.
-
Emerging Role of Cellular Prion Protein in the Maintenance and Expansion of Glioma Stem Cells.Cells. 2019 Nov 18;8(11):1458. doi: 10.3390/cells8111458. Cells. 2019. PMID: 31752162 Free PMC article. Review.
-
Cellular Prion Protein Mediates Pancreatic Cancer Cell Survival and Invasion through Association with and Enhanced Signaling of Notch1.Am J Pathol. 2016 Nov;186(11):2945-2956. doi: 10.1016/j.ajpath.2016.07.010. Epub 2016 Sep 14. Am J Pathol. 2016. PMID: 27639164 Free PMC article.
References
-
- Abujiang P, Mori TJ, Takahashi T, Tanaka F, Kasyu I, Hitomi S, et al. Loss of heterozygosity (LOH) at 17q and 14q in human lung cancers. Oncogene. 1998;17:3029–3033. - PubMed
-
- Adamson R, Jones AS, Field JK. Loss of heterozygosity studies on chromosome 17 in head and neck cancer using microsatellite markers. Oncogene. 1994;9:2077–2082. - PubMed
-
- Akasaka T, van Leeuwen RL, Yoshinaga IG, Mihm MC, Jr, Byers HR. Focal adhesion kinase (p125FAK) expression correlates with motility of human melanoma cell lines. J Invest Dermatol. 1995;105:104–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

