Binding of pro-prion to filamin A: by design or an unfortunate blunder
- PMID: 20697352
- PMCID: PMC3159184
- DOI: 10.1038/onc.2010.307
Binding of pro-prion to filamin A: by design or an unfortunate blunder
Abstract
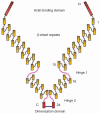
Over the last decades, cancer research has focused on tumor suppressor genes and oncogenes. Genes in other cellular pathways has received less attention. Between 0.5% to 1% of the mammalian genome encodes for proteins that are tethered on the cell membrane via a glycosylphosphatidylinositol (GPI)-anchor. The GPI modification pathway is complex and not completely understood. Prion (PrP), a GPI-anchored protein, is infamous for being the only normal protein that when misfolded can cause and transmit a deadly disease. Though widely expressed and highly conserved, little is known about the functions of PrP. Pancreatic cancer and melanoma cell lines express PrP. However, in these cell lines the PrP exists as a pro-PrP as defined by retaining its GPI anchor peptide signal sequence (GPI-PSS). Unexpectedly, the GPI-PSS of PrP has a filamin A (FLNA) binding motif and binds FLNA. FLNA is a cytolinker protein, and an integrator of cell mechanics and signaling. Binding of pro-PrP to FLNA disrupts the normal FLNA functions. Although normal pancreatic ductal cells lack PrP, about 40% of patients with pancreatic ductal cell adenocarcinoma express PrP in their cancers. These patients have significantly shorter survival time compared with patients whose cancers lack PrP. Pro-PrP is also detected in melanoma in situ but is undetectable in normal melanocyte, and invasive melanoma expresses more pro-PrP. In this review, we will discuss the underlying mechanisms by which binding of pro-PrP to FLNA disrupts normal cellular physiology and contributes to tumorigenesis, and the potential mechanisms that cause the accumulation of pro-PrP in cancer cells.
Figures


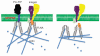
References
-
- Abujiang P, Mori TJ, Takahashi T, Tanaka F, Kasyu I, Hitomi S, et al. Loss of heterozygosity (LOH) at 17q and 14q in human lung cancers. Oncogene. 1998;17:3029–3033. - PubMed
-
- Adamson R, Jones AS, Field JK. Loss of heterozygosity studies on chromosome 17 in head and neck cancer using microsatellite markers. Oncogene. 1994;9:2077–2082. - PubMed
-
- Akasaka T, van Leeuwen RL, Yoshinaga IG, Mihm MC, Jr, Byers HR. Focal adhesion kinase (p125FAK) expression correlates with motility of human melanoma cell lines. J Invest Dermatol. 1995;105:104–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

