Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy
- PMID: 20697374
- PMCID: PMC2991383
- DOI: 10.1038/labinvest.2010.151
Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy
Abstract
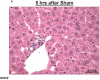
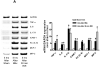
Patients with acute kidney injury (AKI) frequently suffer from extra-renal complications including hepatic dysfunction and systemic inflammation. We aimed to determine the mechanisms of AKI-induced hepatic dysfunction and systemic inflammation. Mice subjected to AKI (renal ischemia reperfusion (IR) or nephrectomy) rapidly developed acute hepatic dysfunction and suffered significantly worse hepatic IR injury. After AKI, rapid peri-portal hepatocyte necrosis, vacuolization, neutrophil infiltration and pro-inflammatory mRNA upregulation were observed suggesting an intestinal source of hepatic injury. Small intestine histology after AKI showed profound villous lacteal capillary endothelial apoptosis, disruption of vascular permeability and epithelial necrosis. After ischemic or non-ischemic AKI, plasma TNF-α, IL-17A and IL-6 increased significantly. Small intestine appears to be the source of IL-17A, as IL-17A levels were higher in the portal circulation and small intestine compared with the levels measured from the systemic circulation and liver. Wild-type mice treated with neutralizing antibodies against TNF-α, IL-17A or IL-6 or mice deficient in TNF-α, IL-17A, IL-17A receptor or IL-6 were protected against hepatic and small intestine injury because of ischemic or non-ischemic AKI. For the first time, we implicate the increased release of IL-17A from small intestine together with induction of TNF-α and IL-6 as a cause of small intestine and liver injury after ischemic or non-ischemic AKI. Modulation of the inflammatory response and cytokine release in the small intestine after AKI may have important therapeutic implications in reducing complications arising from AKI.
Figures






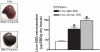



















References
-
- Jones DR, Lee HT. Perioperative renal protection. Best Pract Res Clin Anaesthesiol. 2008;22:193–208. - PubMed
-
- Bove T, Calabro MG, Landoni G, Aletti G, Marino G, Crescenzi G, Rosica C, Zangrillo A. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:442–445. - PubMed
-
- Elapavaluru S, Kellum JA. Why do patients die of acute kidney injury? Acta Clin Belg Suppl. 2007:326–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

