A palladium-catalysed enolate alkylation cascade for the formation of adjacent quaternary and tertiary stereocentres
- PMID: 20697457
- PMCID: PMC2917108
- DOI: 10.1038/nchem.518
A palladium-catalysed enolate alkylation cascade for the formation of adjacent quaternary and tertiary stereocentres
Abstract
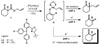
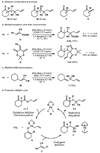
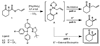
The catalytic enantioselective synthesis of densely functionalised organic molecules containing all-carbon quaternary stereocentres is a challenge to modern chemical methodology research. The catalytically controlled asymmetric alpha-alkylation of ketones represents another difficult task and has been of major interest to our and other research groups in the past. We now report a palladium-catalyzed enantioselective process that addresses both problems at once and allows the installation of vicinal all-carbon quaternary and tertiary stereocentres at the alpha-carbon of a ketone in a single step. This multiple bond forming process is carried out on readily available beta-ketoester starting materials and proceeds via conjugate addition of an in situ-generated palladium enolate to activated Michael acceptors. In other words, the CO(2)-moiety of the substrate is displaced by a C-C fragment in an asymmetric cut-and-paste reaction. The products are obtained in high yield, diastereomeric ratio, and enantiomeric excess.
Figures

Similar articles
-
Catalytic enantioselective construction of quaternary stereocenters: assembly of key building blocks for the synthesis of biologically active molecules.Acc Chem Res. 2015 Mar 17;48(3):740-51. doi: 10.1021/ar5004658. Epub 2015 Feb 25. Acc Chem Res. 2015. PMID: 25715056 Free PMC article.
-
Ligand-enabled multiple absolute stereocontrol in metal-catalysed cycloaddition for construction of contiguous all-carbon quaternary stereocentres.Nat Chem. 2014 Jan;6(1):47-51. doi: 10.1038/nchem.1796. Epub 2013 Nov 24. Nat Chem. 2014. PMID: 24345946
-
Enantioselective Pd-Catalyzed Decarboxylative Allylic Alkylation of Thiopyranones. Access to Acyclic, Stereogenic α-Quaternary Ketones.Org Lett. 2017 Oct 6;19(19):5007-5009. doi: 10.1021/acs.orglett.7b02354. Epub 2017 Sep 13. Org Lett. 2017. PMID: 28901769 Free PMC article.
-
Catalytic asymmetric alkylation of ketones using organometallic reagents.Drug Discov Today Technol. 2013 Spring;10(1):e21-7. doi: 10.1016/j.ddtec.2012.10.010. Drug Discov Today Technol. 2013. PMID: 24050226 Review.
-
Acid-base catalysis of chiral Pd complexes: development of novel catalytic asymmetric reactions and their application to synthesis of drug candidates.Chem Pharm Bull (Tokyo). 2006 Oct;54(10):1351-64. doi: 10.1248/cpb.54.1351. Chem Pharm Bull (Tokyo). 2006. PMID: 17015970 Review.
Cited by
-
Direct Asymmetric Alkylation of Ketones: Still Unconquered.Angew Chem Int Ed Engl. 2017 Aug 1;56(32):9278-9290. doi: 10.1002/anie.201703079. Epub 2017 Jun 27. Angew Chem Int Ed Engl. 2017. PMID: 28497890 Free PMC article. Review.
-
Enantioselective synthesis of α-secondary and α-tertiary piperazin-2-ones and piperazines by catalytic asymmetric allylic alkylation.Angew Chem Int Ed Engl. 2015 Jan 2;54(1):179-83. doi: 10.1002/anie.201408609. Epub 2014 Nov 7. Angew Chem Int Ed Engl. 2015. PMID: 25382664 Free PMC article.
-
Ketone α-alkylation at the more-hindered site.Nat Commun. 2023 Jun 7;14(1):3326. doi: 10.1038/s41467-023-38741-w. Nat Commun. 2023. PMID: 37286579 Free PMC article.
-
Rapid synthesis of an electron-deficient t-BuPHOX ligand: cross-coupling of aryl bromides with secondary phosphine oxides.Tetrahedron Lett. 2010 Oct 20;51(42):5550-5554. doi: 10.1016/j.tetlet.2010.08.039. Tetrahedron Lett. 2010. PMID: 21076623 Free PMC article.
-
Catalytic enantioselective construction of quaternary stereocenters: assembly of key building blocks for the synthesis of biologically active molecules.Acc Chem Res. 2015 Mar 17;48(3):740-51. doi: 10.1021/ar5004658. Epub 2015 Feb 25. Acc Chem Res. 2015. PMID: 25715056 Free PMC article.
References
-
- Das JP, Chechik H, Marek I. A unique approach to aldol products for the creation of all-carbon quaternary stereocenters. Nature Chem. 2009;1:128–132. - PubMed
-
- Christoffers J, Baro A. Quaternary Stereocenters, Challenges and Solutions in Organic Synthesis. Wiley-VCH: Weinheim; 2005.
-
- Trost BM, Jiang C. Catalytic enantioselective construction of all-carbon quaternary stereocenters. Synthesis. 2006:369–396.
-
- Bencivenni G, et al. Targeting structural and stereochemical complexity by organocascade catalysis: Construction of spirocyclic oxindoles having multiple stereocenters. Angew. Chem. Int. Ed. 2009;48:7200–7203. - PubMed
-
- Wu F, Li H, Hong R, Deng L. Construction of quaternary stereocenters by efficient and practical conjugate additions to α,β-unsaturated ketones with a chiral organic catalyst. Angew. Chem. Int. Ed. 2006;45:947–950. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

