Designing multifunctional quantum dots for bioimaging, detection, and drug delivery
- PMID: 20697629
- PMCID: PMC3212036
- DOI: 10.1039/b915139g
Designing multifunctional quantum dots for bioimaging, detection, and drug delivery
Abstract
The emerging field of bionanotechnology aims at revolutionizing biomedical research and clinical practice via introduction of nanoparticle-based tools, expanding capabilities of existing investigative, diagnostic, and therapeutic techniques as well as creating novel instruments and approaches for addressing challenges faced by medicine. Quantum dots (QDs), semiconductor nanoparticles with unique photo-physical properties, have become one of the dominant classes of imaging probes as well as universal platforms for engineering of multifunctional nanodevices. Possessing versatile surface chemistry and superior optical features, QDs have found initial use in a variety of in vitro and in vivo applications. However, careful engineering of QD probes guided by application-specific design criteria is becoming increasingly important for successful transition of this technology from proof-of-concept studies towards real-life clinical applications. This review outlines the major design principles and criteria, from general ones to application-specific, governing the engineering of novel QD probes satisfying the increasing demands and requirements of nanomedicine and discusses the future directions of QD-focused bionanotechnology research (critical review, 201 references).
Figures



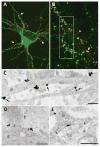
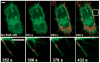






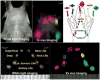







References
-
- Nanoscale Science Engineering and Technology Subcommittee (NSET), Committee on Technology (CT) and National Science and Technology Council (NSTC) Vol. 44 2009.
-
- Chen H, Roco MC, Li X, Lin Y. Nat Nanotechnol. 2008;3:123–125. - PubMed
-
- Massoud TF, Gambhir SS. Genes & development. 2003;17:545–580. - PubMed
-
- Mankoff DA. J Nucl Med. 2007;48:18N–21N. - PubMed
-
- Medintz IL, Mattoussi H. Phys Chem Chem Phys. 2009;11:17–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

