Endothelial nitric oxide synthase transgenic models of endothelial dysfunction
- PMID: 20697735
- PMCID: PMC2975487
- DOI: 10.1007/s00424-010-0867-4
Endothelial nitric oxide synthase transgenic models of endothelial dysfunction
Abstract
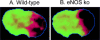
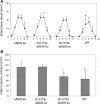
Endothelial production of nitric oxide is critical to the regulation of vascular responses, including vascular tone and regional blood flow, leukocyte-endothelial interactions, platelet adhesion and aggregation, and vascular smooth muscle cell proliferation. A relative deficiency in the amount of bioavailable vascular NO results in endothelial dysfunction, with conditions that are conducive to the development of atherosclerosis: thrombosis, inflammation, neointimal proliferation, and vasoconstriction. This review focuses on mouse models of endothelial dysfunction caused by direct genetic modification of the endothelial nitric oxide synthase (eNOS) gene. We first describe the cardiovascular phenotypes of eNOS knockout mice, which are a model of total eNOS gene deficiency and thus the ultimate model of endothelial dysfunction. We then describe S1177A and S1177D eNOS mutant mice as mouse models with altered eNOS phosphorylation and therefore varying degrees of endothelial dysfunction. These include transgenic mice that carry the eNOS S1177A and S1177D transgenes, as well as knockin mice in which the endogenous eNOS gene has been mutated to carry the S1177A and S1177D mutations. Together, eNOS knockout mice and eNOS S1177 mutant mice are useful tools to study the effects of total genetic deficiency of eNOS as well as varying degrees of endothelial dysfunction caused by eNOS S1177 phosphorylation.
Figures



References
-
- Gimbrone MA., Jr Endothelial dysfunction and atherosclerosis. J Card Surg. 1989;4:180–183. - PubMed
-
- Gimbrone MA., Jr Vascular endothelium: an integrator of pathophysiologic stimuli in atherosclerosis. Am J Cardiol. 1995;75:67B–70B. - PubMed
-
- Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann NYAcad Sci. 2000;902:230–239. discussion 239–40. - PubMed
-
- Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419–S420. - PubMed
-
- Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

