(+)-Methamphetamine-induced monoamine reductions and impaired egocentric learning in adrenalectomized rats is independent of hyperthermia
- PMID: 20698032
- PMCID: PMC2921964
- DOI: 10.1002/syn.20784
(+)-Methamphetamine-induced monoamine reductions and impaired egocentric learning in adrenalectomized rats is independent of hyperthermia
Abstract
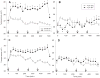
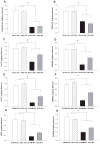
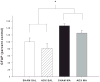
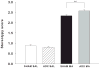
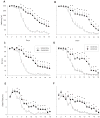
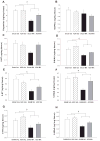
Methamphetamine (MA) is widely abused and implicated in residual cognitive deficits. In rats, increases in plasma corticosterone and egocentric learning deficits are observed after a 1-day binge regimen of MA (10 mg/kg x 4 at 2-h intervals). The purpose of this experiment was to determine if adrenal inactivation during and following MA exposure would attenuate the egocentric learning deficits in the Cincinnati water maze (CWM). In the first experiment, the effects of adrenalectomy (ADX) or sham surgery (SHAM) on MA-induced neurotoxicity at 72 h were determined. SHAM-MA animals showed typical patterns of hyperthermia, whereas ADX-MA animals were normothermic. Both SHAM-MA- and ADX-MA-treated animals showed increased neostriatal glial fibrillary acidic protein and decreased monoamines in the neostriatum, hippocampus, and entorhinal cortex. In the second experiment, SHAM-MA- and ADX-MA-treated groups showed equivalently impaired CWM performance 2 weeks post-treatment (increased latencies, errors, and start returns) compared to SHAM-saline (SAL) and ADX-SAL groups with no effects on novel object recognition, elevated zero maze, or acoustic startle/prepulse inhibition. Post-testing, monoamine levels remained decreased in both MA-treated groups in all three brain regions, but were not as large as those observed at 72-h post-treatment. The data demonstrate that MA-induced learning deficits can be dissociated from drug-induced increases in plasma corticosterone or hyperthermia, but co-occur with dopamine and serotonin reductions.
(c) 2010 Wiley-Liss, Inc.
Figures
Similar articles
-
Effect of a neurotoxic dose regimen of (+)-methamphetamine on behavior, plasma corticosterone, and brain monoamines in adult C57BL/6 mice.Neurotoxicol Teratol. 2010 May-Jun;32(3):346-55. doi: 10.1016/j.ntt.2010.01.006. Epub 2010 Jan 21. Neurotoxicol Teratol. 2010. PMID: 20096350 Free PMC article.
-
Effect of +-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats.Psychopharmacology (Berl). 2008 Sep;199(4):637-50. doi: 10.1007/s00213-008-1183-y. Epub 2008 May 29. Psychopharmacology (Berl). 2008. PMID: 18509623 Free PMC article.
-
Effects of intrastriatal dopamine D1 or D2 antagonists on methamphetamine-induced egocentric and allocentric learning and memory deficits in Sprague-Dawley rats.Psychopharmacology (Berl). 2019 Jul;236(7):2243-2258. doi: 10.1007/s00213-019-05221-3. Epub 2019 Mar 27. Psychopharmacology (Berl). 2019. PMID: 30919007 Free PMC article.
-
Methamphetamine-induced toxicity: an updated review on issues related to hyperthermia.Pharmacol Ther. 2014 Oct;144(1):28-40. doi: 10.1016/j.pharmthera.2014.05.001. Epub 2014 May 14. Pharmacol Ther. 2014. PMID: 24836729 Free PMC article. Review.
-
A Review on the Disruption of Novel Object Recognition Induced by Methamphetamine.Addict Health. 2023 Oct;15(4):289-297. doi: 10.34172/ahj.2023.1307. Epub 2023 Oct 29. Addict Health. 2023. PMID: 38322487 Free PMC article. Review.
Cited by
-
Nucleus accumbens invulnerability to methamphetamine neurotoxicity.ILAR J. 2011;52(3):352-65. doi: 10.1093/ilar.52.3.352. ILAR J. 2011. PMID: 23382149 Free PMC article. Review.
-
Involvement of the α(1D)-adrenergic receptor in methamphetamine-induced hyperthermia and neurotoxicity in rats.Neurotox Res. 2013 Aug;24(2):130-8. doi: 10.1007/s12640-012-9369-9. Epub 2013 Jan 3. Neurotox Res. 2013. PMID: 23283760
-
Long-term effects of exposure to methamphetamine in adolescent rats.Drug Alcohol Depend. 2014 May 1;138:17-23. doi: 10.1016/j.drugalcdep.2014.02.021. Epub 2014 Feb 26. Drug Alcohol Depend. 2014. PMID: 24629630 Free PMC article.
-
Cincinnati water maze: A review of the development, methods, and evidence as a test of egocentric learning and memory.Neurotoxicol Teratol. 2016 Sep-Oct;57:1-19. doi: 10.1016/j.ntt.2016.08.002. Epub 2016 Aug 18. Neurotoxicol Teratol. 2016. PMID: 27545092 Free PMC article. Review.
-
Comparison of (+)-methamphetamine, ±-methylenedioxymethamphetamine, (+)-amphetamine and ±-fenfluramine in rats on egocentric learning in the Cincinnati water maze.Synapse. 2011 May;65(5):368-78. doi: 10.1002/syn.20854. Epub 2010 Oct 8. Synapse. 2011. PMID: 20730798 Free PMC article.
References
-
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–1114. - PubMed
-
- Ali SF, Newport GD, Holson RR, Slikker W, Jr, Bowyer JF. Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res. 1994;658:33–38. - PubMed
-
- Baicy K, London ED. Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction. 2007;102(Suppl 1):5–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
