Vaccine delivery by polymeric vehicles in the mouse reproductive tract induces sustained local and systemic immunity
- PMID: 20698574
- PMCID: PMC3004023
- DOI: 10.1021/mp100009e
Vaccine delivery by polymeric vehicles in the mouse reproductive tract induces sustained local and systemic immunity
Abstract
Design of easily administered vaccines to protect the female reproductive tract against STIs such as HIV, HPV and HSV is a major step in improving world health standards. However, the effect of immunization routes and regimens (prime/boost) on immune response is not well-understood. Here, we present a systematic study of vaccine delivery by different routes and prime/boosting regimens to produce a robust humoral immune response in the reproductive tract. A model antigen, ovalbumin (OVA), was delivered orally or intranasally via polymer particles, and intravaginally via polymer disks to female mice. Repeated prime/boost at a single site result in high OVA-specific antibody levels in the serum for mice immunized orally (IgA) and invaginally (IgA and IgG) after 3 months. Vaginal antibody titers were the highest for mice immunized by intravaginal routes. Vaginal boosting following intranasal or oral priming did not appear to offer similar advantages to those primed intravaginally. Systemic immunization with OVA in Freund's adjuvant produced robust serum IgG levels, but little serum IgA or antibodies in the vaginal washings. All immunization schemes produced a significant level of IgG in the intestinal mucosa, with the exception of nasal priming followed by intravaginal boost with slow-releasing disks. In contrast, only immunization by nasal priming and intravaginal boost with fast-releasing disks was able to achieve significantly high intestinal IgA titers.
Figures




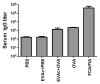
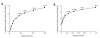
Similar articles
-
Per-oral immunization with antigen-conjugated nanoparticles followed by sub-cutaneous boosting immunization induces long-lasting mucosal and systemic antibody responses in mice.PLoS One. 2015 Feb 24;10(2):e0118067. doi: 10.1371/journal.pone.0118067. eCollection 2015. PLoS One. 2015. PMID: 25710518 Free PMC article.
-
Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant.Infect Immun. 1996 Mar;64(3):974-9. doi: 10.1128/iai.64.3.974-979.1996. Infect Immun. 1996. PMID: 8641809 Free PMC article.
-
Evaluation of mucosal and systemic immune responses elicited by GPI-0100- adjuvanted influenza vaccine delivered by different immunization strategies.PLoS One. 2013 Jul 31;8(7):e69649. doi: 10.1371/journal.pone.0069649. Print 2013. PLoS One. 2013. PMID: 23936066 Free PMC article.
-
Enhanced mucosal and systemic immune responses following intravaginal immunization with human papillomavirus 16 L1 virus-like particle vaccine in thermosensitive mucoadhesive delivery systems.J Med Virol. 2003 Aug;70(4):633-41. doi: 10.1002/jmv.10442. J Med Virol. 2003. PMID: 12794729
-
Intranasal HIV-1-gp160-DNA/gp41 peptide prime-boost immunization regimen in mice results in long-term HIV-1 neutralizing humoral mucosal and systemic immunity.J Immunol. 2004 Dec 1;173(11):7078-89. doi: 10.4049/jimmunol.173.11.7078. J Immunol. 2004. PMID: 15557206
Cited by
-
Biodistribution of Polymeric Nanoparticles following in utero Delivery to a Nonhuman Primate.Biomed Hub. 2024 Dec 12;10(1):23-32. doi: 10.1159/000543138. eCollection 2025 Jan-Dec. Biomed Hub. 2024. PMID: 39845408 Free PMC article.
-
Demystifying particle-based oral vaccines.Expert Opin Drug Deliv. 2021 Oct;18(10):1455-1472. doi: 10.1080/17425247.2021.1946511. Epub 2021 Jul 6. Expert Opin Drug Deliv. 2021. PMID: 34148474 Free PMC article. Review.
-
Protein and oligonucleotide delivery systems for vaginal microbicides against viral STIs.Cell Mol Life Sci. 2015 Feb;72(3):469-503. doi: 10.1007/s00018-014-1756-3. Epub 2014 Oct 17. Cell Mol Life Sci. 2015. PMID: 25323132 Free PMC article. Review.
-
Efficacy of polymeric encapsulated C5a peptidase-based group B streptococcus vaccines in a murine model.Am J Obstet Gynecol. 2011 Sep;205(3):249.e1-8. doi: 10.1016/j.ajog.2011.06.024. Epub 2011 Jun 15. Am J Obstet Gynecol. 2011. PMID: 21802065 Free PMC article.
-
Recent Advances in Polymer-Based Vaginal Drug Delivery Systems.Pharmaceutics. 2021 Jun 15;13(6):884. doi: 10.3390/pharmaceutics13060884. Pharmaceutics. 2021. PMID: 34203714 Free PMC article. Review.
References
-
- Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. - PubMed
-
- Walker RI. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine. 1994;12(5):387–400. - PubMed
-
- UNAIDS . In: Global summary of the AIDS epidemic. Organization WH, editor. 2007.
-
- Harper DM. Preliminary HPV vaccine results for women older than 25 years. Lancet. 2009;373(9679):1921–1922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

