Human serum processing and analysis methods for rapid and reproducible N-glycan mass profiling
- PMID: 20698584
- PMCID: PMC2948646
- DOI: 10.1021/pr100202a
Human serum processing and analysis methods for rapid and reproducible N-glycan mass profiling
Abstract
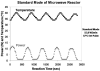
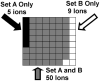
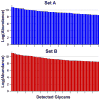
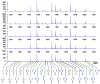
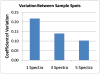
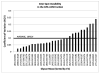
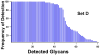
Glycans constitute a new class of compounds for biomarker discovery. Glycosylation is a common post-translational modification and is often associated with transformation to malignancy. To analyze glycans, they are released from proteins, enriched, and measured with mass spectrometry. For biomarker discovery, repeatability at every step of the process is important. Locating and minimizing the process variability is key to establishing a robust platform stable enough for biomarker discovery. Understanding the variability of the measurement devices helps understand the variability associated with the chemical processing. This report explores the potential use of methods expediting the enzymatic release of glycans such as a microwave reactor and automation of the solid-phase extraction with a robotic liquid handler. The study employs matrix-assisted laser desorption/ionization-Fourier transform ion cyclotron resonance mass spectrometry but would be suitable with any mass spectrometry method. Methods for system-wide data analysis are examined because proper metrics for evaluating the performance of glycan sample preparation procedures are not well established.
Figures


References
-
- Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473(1):4–8. - PubMed
-
- Vance BA, Wu WY, Ribaudo RK, Segal DM, Kearse KP. Multiple dimeric forms of human CD69 result from differential addition of N-glycans to typical (Asn-X-Ser/Thr) and atypical (Asn-X-Cys) glycosylation motifs. Journal of Biological Chemistry. 1997;272(37):23117–23122. - PubMed
-
- Miletich JP, Broze GJ. Beta-Protein-C Is Not Glycosylated at Asparagine-329 - the Rate of Translation May Influence the Frequency of Usage at Asparagine-X-Cysteine Sites. Journal of Biological Chemistry. 1990;265(19):11397–11404. - PubMed
-
- Titani K, Kumar S, Takio K, Ericsson LH, Wade RD, Ashida K, Walsh KA, Chopek MW, Sadler JE, Fujikawa K. Amino-Acid-Sequences of Human Vonwillebrand-Factor. Biochemistry. 1986;25(11):3171–3184. - PubMed
-
- Lebrilla CB, An HJ. The prospects of glycan biomarkers for the diagnosis of diseases. Mol Biosyst. 2009;5(1):17–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

