Magic angle spinning NMR investigation of influenza A M2(18-60): support for an allosteric mechanism of inhibition
- PMID: 20698642
- PMCID: PMC2922979
- DOI: 10.1021/ja101537p
Magic angle spinning NMR investigation of influenza A M2(18-60): support for an allosteric mechanism of inhibition
Abstract
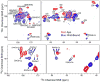
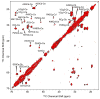
The tetrameric M2 proton channel from influenza A virus conducts protons at low pH and is inhibited by aminoadamantyl drugs such as amantadine and rimantadine (Rmt). We report magic angle spinning NMR spectra of POPC and DPhPC membrane-embedded M2(18-60), both apo and in the presence of Rmt. Similar line widths in the spectra of apo and bound M2 indicate that Rmt does not have a significant impact on the dynamics or conformational heterogeneity of this construct. Substantial chemical shift changes for many residues in the transmembrane region support an allosteric mechanism of inhibition. An Rmt titration supports a binding stoichiometry of >1 Rmt molecule per channel and shows that nonspecific binding or changes in membrane composition are unlikely sources of the chemical shift changes. In addition, doubling of spectral lines in all of the observed samples provides evidence that the channel assembles with twofold symmetry.
Figures


References
-
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. JAMA. 2006;295:891–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

