Enantioselective synthesis of axially chiral biaryls by the Pd-catalyzed Suzuki-Miyaura reaction: substrate scope and quantum mechanical investigations
- PMID: 20698695
- PMCID: PMC2924746
- DOI: 10.1021/ja104297g
Enantioselective synthesis of axially chiral biaryls by the Pd-catalyzed Suzuki-Miyaura reaction: substrate scope and quantum mechanical investigations
Abstract
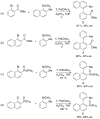
We report efficient syntheses of axially chiral biaryl amides in yields ranging from 80-92%, and with enantioselectivity in the range 88-94% ee employing an asymmetric Suzuki-Miyaura process with Pd(OAc)(2) and KenPhos as ligand. These studies demonstrate that electron-rich and electron-deficient o-halobenzamides can be efficiently coupled with 2-methyl-1-naphthylboronic acid and 2-ethoxy-1-naphthylboronic acid. The yields and selectivities of the reactions are independent of the nature of halogen substituent on the benzamide coupling partner. Our investigations demonstrate that axially chiral heterocyclic and biphenyl compounds can also be synthesized with this methodology. We also report computational studies used to determine the origin of stereoselectivity during the selectivity-determining reductive elimination step of the related coupling of tolyl boronic acid with naphthylphosphonate bromide that was reported in a previous publication (J. Am. Chem. Soc. 2000, 122, 12051-12052). These studies indicate that the stereoselectivity arises from a combination of weak -(C)H..O interactions as well as steric interactions between the tolyl and naphthylphosphonate addends in the transition state for C-C coupling.
Figures












References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

