Human adipose-derived stromal cells stimulate autogenous skeletal repair via paracrine Hedgehog signaling with calvarial osteoblasts
- PMID: 20698749
- PMCID: PMC3128781
- DOI: 10.1089/scd.2010.0250
Human adipose-derived stromal cells stimulate autogenous skeletal repair via paracrine Hedgehog signaling with calvarial osteoblasts
Abstract
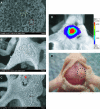
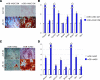
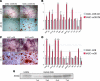
Human adipose-derived stromal cells (hASCs) have the proven capacity to ossify skeletal defects. The mechanisms whereby hASCs stimulate bone repair are not fully understood. In this study, we examined the potential for hASCs to stimulate autogenous repair of a mouse calvarial defect. Immunofluoresence, osteogenic stains, and surface electron microscopy were used to demonstrate osteogenic differentiation of hASCs. hASCs were engrafted into 4 mm calvarial defects in athymic mice using an osteoconductive scaffold. Analysis included microcomputed tomography, histology, in situ hybridization, and quantitative real-time-polymerase chain reaction. Next, the in vitro interaction between hASCs and mouse calvarial osteoblasts (mOBs) was assessed by the conditioned medium and coculture assays. The medium was supplemented with Hedgehog signaling modifiers, including recombinant N-terminal Sonic hedgehog, smoothened agonist, and cyclopamine. Finally, cyclopamine was delivered in vivo to hASC-engrafted defects. Significant calvarial healing was observed among hASC-engrafted defects compared with control groups (no treatment or scaffold alone) (*P<0.05). hASCs showed evidence of stimulation of host mouse osteogenesis, including (1) increased expression of bone markers at the defect edge by in situ hybridization, and (2) increased host osteogenic gene expression by species-specific quantitative real-time polymerase chain reaction. Using the conditioned medium or coculture assays, hASCs stimulated mOB osteogenic differentiation, accompanied by Hedgehog signaling activation. N-terminal Sonic hedgehog or smoothened agonist replicated, while cyclopamine reversed, the pro-osteogenic effect of the conditioned medium on mOBs. Finally, cyclopamine injection arrested bone formation in vivo. hASCs heal critical-sized mouse calvarial defects, this is, at least in part, via stimulation of autogenous healing of the host defect. Our studies suggest that hASC-derived Hedgehog signaling may play a paracrine role in skeletal repair.
Figures




References
-
- HCUP. Agency for Healthcare Research and Quality; 2007. Healthcare cost and utilization project. - PubMed
-
- Fong KD. Nacamuli RP. Song HM. Warren SM. Lorenz HP. Longaker MT. New strategies for craniofacial repair and replacement: a brief review. J Craniofac Surg. 2003;14:333–339. - PubMed
-
- Nacamuli RP. Longaker MT. Bone induction in craniofacial defects. Orthod Craniofac Res. 2005;8:259–266. - PubMed
-
- Halvorsen YC. Wilkison WO. Gimble JM. Adipose-derived stromal cells—their utility and potential in bone formation. Int J Obes Relat Metab Disord. 2000;24(Suppl 4):S41–S44. - PubMed
-
- Lendeckel S. Jodicke A. Christophis P. Heidinger K. Wolff J. Fraser JK. Hedrick MH. Berthold L. Howaldt HP. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

