Vascular endothelial growth factor and spinal cord injury pain
- PMID: 20698758
- PMCID: PMC2953928
- DOI: 10.1089/neu.2010.1351
Vascular endothelial growth factor and spinal cord injury pain
Abstract
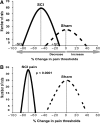
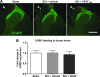
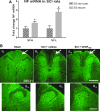
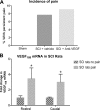
Vascular endothelial growth factor (VEGF)-A mRNA was previously identified as one of the significantly upregulated transcripts in spinal cord injured tissue from adult rats that developed allodynia. To characterize the role of VEGF-A in the development of pain in spinal cord injury (SCI), we analyzed mechanical allodynia in SCI rats that were treated with either vehicle, VEGF-A isoform 165 (VEGF(165)), or neutralizing VEGF(165)-specific antibody. We have observed that exogenous administration of VEGF(165) increased both the number of SCI rats that develop persistent mechanical allodynia, and the level of hypersensitivity to mechanical stimuli. Our analysis identified excessive and aberrant growth of myelinated axons in dorsal horns and dorsal columns of chronically injured spinal cords as possible mechanisms for both SCI pain and VEGF(165)-induced amplification of SCI pain, suggesting that elevated endogenous VEGF(165) may have a role in the development of allodynia after SCI. However, the neutralizing VEGF(165) antibody showed no effect on allodynia or axonal sprouting after SCI. It is possible that another endogenous VEGF isoform activates the same signaling pathway as the exogenously-administered 165 isoform and contributes to SCI pain. Our transcriptional analysis revealed that endogenous VEGF(188) is likely to be the isoform involved in the development of allodynia after SCI. To the best of our knowledge, this is the first study to suggest a possible link between VEGF, nonspecific sprouting of myelinated axons, and mechanical allodynia following SCI.
Figures


Similar articles
-
Low-energy extracorporeal shock wave therapy for promotion of vascular endothelial growth factor expression and angiogenesis and improvement of locomotor and sensory functions after spinal cord injury.J Neurosurg Spine. 2016 Dec;25(6):745-755. doi: 10.3171/2016.4.SPINE15923. Epub 2016 Jul 1. J Neurosurg Spine. 2016. PMID: 27367940
-
Upregulation of calcium channel alpha-2-delta-1 subunit in dorsal horn contributes to spinal cord injury-induced tactile allodynia.Spine J. 2018 Jun;18(6):1062-1069. doi: 10.1016/j.spinee.2018.01.010. Epub 2018 Jan 31. Spine J. 2018. PMID: 29355786
-
Repetitive intrathecal VEGF(165) treatment has limited therapeutic effects after spinal cord injury in the rat.J Neurotrauma. 2010 Oct;27(10):1781-91. doi: 10.1089/neu.2010.1484. J Neurotrauma. 2010. PMID: 20701430
-
Mechanisms of chronic central neuropathic pain after spinal cord injury.Brain Res Rev. 2009 Apr;60(1):202-13. doi: 10.1016/j.brainresrev.2008.12.010. Epub 2008 Dec 25. Brain Res Rev. 2009. PMID: 19154757 Free PMC article. Review.
-
The Impact of Activity-Based Interventions on Neuropathic Pain in Experimental Spinal Cord Injury.Cells. 2022 Sep 30;11(19):3087. doi: 10.3390/cells11193087. Cells. 2022. PMID: 36231048 Free PMC article. Review.
Cited by
-
Delayed administration of a bio-engineered zinc-finger VEGF-A gene therapy is neuroprotective and attenuates allodynia following traumatic spinal cord injury.PLoS One. 2014 May 20;9(5):e96137. doi: 10.1371/journal.pone.0096137. eCollection 2014. PLoS One. 2014. PMID: 24846143 Free PMC article.
-
VEGFR2 promotes central endothelial activation and the spread of pain in inflammatory arthritis.Brain Behav Immun. 2018 Nov;74:49-67. doi: 10.1016/j.bbi.2018.03.012. Epub 2018 Mar 14. Brain Behav Immun. 2018. PMID: 29548992 Free PMC article.
-
Electroacupuncture improves thermal and mechanical sensitivities in a rat model of postherpetic neuralgia.Mol Pain. 2013 Apr 3;9:18. doi: 10.1186/1744-8069-9-18. Mol Pain. 2013. PMID: 23551937 Free PMC article.
-
Netrin-1 Contributes to Myelinated Afferent Fiber Sprouting and Neuropathic Pain.Mol Neurobiol. 2016 Oct;53(8):5640-51. doi: 10.1007/s12035-015-9482-x. Epub 2015 Oct 19. Mol Neurobiol. 2016. PMID: 26482371
-
IL-17 induces reactive astrocytes and up-regulation of vascular endothelial growth factor (VEGF) through JAK/STAT signaling.Sci Rep. 2017 Mar 10;7:41779. doi: 10.1038/srep41779. Sci Rep. 2017. PMID: 28281545 Free PMC article.
References
-
- Ackery A.D. Norenberg M.D. Krassioukov A. Calcitonin gene-related peptide immunoreactivity in chronic human spinal cord injury. Spinal Cord. 2007;45:678–686. - PubMed
-
- Agudo M. Robinson M. Cafferty W. Bradbury E.J. Kilkenny C. Hunt S.P. McMahon S.B. Regulation of neuropilin 1 by spinal cord injury in adult rats. Mol. Cell Neurosci. 2005;28:475–484. - PubMed
-
- Baastrup C. Finnerup N.B. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs. 2008;22:455–475. - PubMed
-
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. - PubMed
-
- Bian D. Ossipov M.H. Zhong C. Malan T.P., Jr. Porreca F. Tactile allodynia, but not thermal hyperalgesia, of the hindlimbs is blocked by spinal transection in rats with nerve injury. Neurosci. Lett. 1998;241:79–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

