Neuroproteomics: a biochemical means to discriminate the extent and modality of brain injury
- PMID: 20698760
- PMCID: PMC2953930
- DOI: 10.1089/neu.2010.1374
Neuroproteomics: a biochemical means to discriminate the extent and modality of brain injury
Abstract
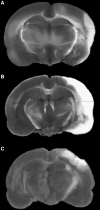
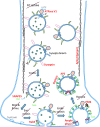
Diagnosis and treatment of stroke and traumatic brain injury remain significant health care challenges to society. Patient care stands to benefit from an improved understanding of the interactive biochemistry underlying neurotrauma pathobiology. In this study, we assessed the power of neuroproteomics to contrast biochemical responses following ischemic and traumatic brain injuries in the rat. A middle cerebral artery occlusion (MCAO) model was employed in groups of 30-min and 2-h focal neocortical ischemia with reperfusion. Neuroproteomes were assessed via tandem cation-anion exchange chromatography-gel electrophoresis, followed by reversed-phase liquid chromatography-tandem mass spectrometry. MCAO results were compared with those from a previous study of focal contusional brain injury employing the same methodology to characterize homologous neocortical tissues at 2 days post-injury. The 30-min MCAO neuroproteome depicted abridged energy production involving pentose phosphate, modulated synaptic function and plasticity, and increased chaperone activity and cell survival factors. The 2-h MCAO data indicated near complete loss of ATP production, synaptic dysfunction with degraded cytoarchitecture, more conservative chaperone activity, and additional cell survival factors than those seen in the 30-min MCAO model. The TBI group exhibited disrupted metabolism, but with retained malate shuttle functionality. Synaptic dysfunction and cytoarchitectural degradation resembled the 2-h MCAO group; however, chaperone and cell survival factors were more depressed following TBI. These results underscore the utility of neuroproteomics for characterizing interactive biochemistry for profiling and contrasting the molecular aspects underlying the pathobiological differences between types of brain injuries.
Figures


Similar articles
-
Proteomic Analysis of Rat Cerebral Cortex in the Subacute to Long-Term Phases of Focal Cerebral Ischemia-Reperfusion Injury.J Proteome Res. 2019 Aug 2;18(8):3099-3118. doi: 10.1021/acs.jproteome.9b00220. Epub 2019 Jul 2. J Proteome Res. 2019. PMID: 31265301
-
Novel differential neuroproteomics analysis of traumatic brain injury in rats.Mol Cell Proteomics. 2006 Oct;5(10):1887-98. doi: 10.1074/mcp.M600157-MCP200. Epub 2006 Jun 26. Mol Cell Proteomics. 2006. PMID: 16801361
-
Combined metabolic and transcriptional profiling identifies pentose phosphate pathway activation by HSP27 phosphorylation during cerebral ischemia.Neuroscience. 2017 May 4;349:1-16. doi: 10.1016/j.neuroscience.2017.02.036. Epub 2017 Mar 6. Neuroscience. 2017. PMID: 28257891
-
Neuroprotection by mesenchymal stem cell (MSC) administration is enhanced by local cooling infusion (LCI) in ischemia.Brain Res. 2019 Dec 1;1724:146406. doi: 10.1016/j.brainres.2019.146406. Epub 2019 Aug 24. Brain Res. 2019. PMID: 31454517 Review.
-
Neuroproteome Dynamics in Modeled Brain Injury: A Systems Neurobiology Perspective.In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 27. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 27. PMID: 26269918 Free Books & Documents. Review.
Cited by
-
Anaplasma phagocytophilum Asp14 is an invasin that interacts with mammalian host cells via its C terminus to facilitate infection.Infect Immun. 2013 Jan;81(1):65-79. doi: 10.1128/IAI.00932-12. Epub 2012 Oct 15. Infect Immun. 2013. PMID: 23071137 Free PMC article.
-
Integration of proteomics, bioinformatics, and systems biology in traumatic brain injury biomarker discovery.Front Neurol. 2013 May 31;4:61. doi: 10.3389/fneur.2013.00061. eCollection 2013. Front Neurol. 2013. PMID: 23750150 Free PMC article.
-
Stroke Proteomics: From Discovery to Diagnostic and Therapeutic Applications.Circ Res. 2022 Apr 15;130(8):1145-1166. doi: 10.1161/CIRCRESAHA.122.320110. Epub 2022 Apr 14. Circ Res. 2022. PMID: 35420912 Free PMC article. Review.
-
Co-Expression Network Analysis of MicroRNAs and Proteins in Severe Traumatic Brain Injury: A Systematic Review.Cells. 2021 Sep 14;10(9):2425. doi: 10.3390/cells10092425. Cells. 2021. PMID: 34572074 Free PMC article.
-
Temporal Genetic Modifications after Controlled Cortical Impact--Understanding Traumatic Brain Injury through a Systematic Network Approach.Int J Mol Sci. 2016 Feb 6;17(2):216. doi: 10.3390/ijms17020216. Int J Mol Sci. 2016. PMID: 26861311 Free PMC article.
References
-
- Agoston D.V. Gyorgy A. Eidelman O. Pollard H.B. Proteomic biomarkers for blast neurotrauma: targeting cerebral edema, inflammation, and neuronal death cascades. J. Neurotrauma. 2009;26:901–911. - PubMed
-
- Baird A.E. Blood biologic markers of stroke: improved management, reduced cost? Curr. Atheroscler. Rep. 2006;8:267–275. - PubMed
-
- Baron J.C. Perfusion thresholds in human cerebral ischemia: historical perspective and therapeutic implications. Cerebrovasc. Dis. 2001;11(Suppl. 1):2–8. - PubMed
-
- Berger R.P. Beers S.R. Richichi R. Wiesman D. Adelson P.D. Serum biomarker concentrations and outcome after pediatric traumatic brain injury. J. Neurotrauma. 2007;24:1793–1801. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

