Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons
- PMID: 20699362
- PMCID: PMC2996899
- DOI: 10.1189/jlb.0610318
Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons
Abstract
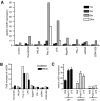
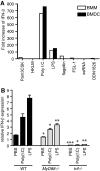
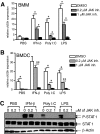
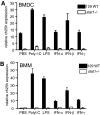
The oxysterol-producing enzyme CH25H plays an important role in regulating lipid metabolism, gene expression, and immune activation. In vitro experiments using a panel of TLR agonists to activate BMDCs and macrophages demonstrated that Ch25h expression is induced rapidly, selectively, and robustly by the TLR ligands poly I:C and LPS. The mechanism of TLR3- and TLR4-induced transcription levels of Ch25h relies on the TRIF-mediated production of type I IFNs and requires signaling through the IFNαR and JAK/STAT1 pathway. Treatment of BMDCs and macrophages with IFN-α or IFN-β induces Ch25h in a STAT1-dependent manner. IFN-γ also up-regulated Ch25h expression by signaling through STAT1, suggesting that multiple pathways regulate the production of this enzyme. In addition, we demonstrated that regulation of Ch25h expression in vivo in lung-derived DCs and macrophages is dependent on signaling through the IFNαR and STAT1. The results suggest that the rapid induction of Ch25h and subsequent oxysterol synthesis may represent a component of the regulatory network that modulates the magnitude of innate immune reactions and possibly the nature and intensity of subsequent adaptive responses.
Figures

Comment in
-
Editorial: 25-Hydroxycholesterol: a new life in immunology.J Leukoc Biol. 2010 Dec;88(6):1071-2. doi: 10.1189/jlb.0710418. J Leukoc Biol. 2010. PMID: 21123296 Free PMC article.
References
-
- Russell D. W. (2000) Oxysterol biosynthetic enzymes. Biochim. Biophys. Acta 1529, 126–135 - PubMed
-
- Lehmann J. M., Kliewer S. A., Moore L. B., Smith-Oliver T. A., Oliver B. B., Su J. L., Sundseth S. S., Winegar D. A., Blanchard D. E., Spencer T. A., Willson T. M. (1997) Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 272, 3137–3140 - PubMed
-
- Nes W. D., Lukyanenko Y. O., Jia Z. H., Quideau S., Howald W. N., Pratum T. K., West R. R., Hutson J. C. (2000) Identification of the lipophilic factor produced by macrophages that stimulates steroidogenesis. Endocrinology 141, 953–958 - PubMed
-
- Brown M. S., Goldstein J. L. (1997) The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–340 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

