Analysis of proteome dynamics in the mouse brain
- PMID: 20699386
- PMCID: PMC2922600
- DOI: 10.1073/pnas.1006551107
Analysis of proteome dynamics in the mouse brain
Erratum in
- Proc Natl Acad Sci U S A. 2014 Mar 4;111(9):3645
Abstract
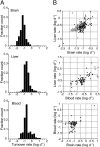
Advances in systems biology have allowed for global analyses of mRNA and protein expression, but large-scale studies of protein dynamics and turnover have not been conducted in vivo. Protein turnover is an important metabolic and regulatory mechanism in establishing proteome homeostasis, impacting many physiological and pathological processes. Here, we have used organism-wide isotopic labeling to measure the turnover rates of approximately 2,500 proteins in multiple mouse tissues, spanning four orders of magnitude. Through comparison of the brain with the liver and blood, we show that within the respective tissues, proteins performing similar functions often have similar turnover rates. Proteins in the brain have significantly slower turnover (average lifetime of 9.0 d) compared with those of the liver (3.0 d) and blood (3.5 d). Within some organelles (such as mitochondria), proteins have a narrow range of lifetimes, suggesting a synchronized turnover mechanism. Protein subunits within complexes of variable composition have a wide range of lifetimes, whereas those within well-defined complexes turn over in a coordinated manner. Together, the data represent the most comprehensive in vivo analysis of mammalian proteome turnover to date. The developed methodology can be adapted to assess in vivo proteome homeostasis in any model organism that will tolerate a labeled diet and may be particularly useful in the analysis of neurodegenerative diseases in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Goldberg AL, St John AC. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. - PubMed
-
- Mortimore GE, Pösö AR, Lardeux BR. Mechanism and regulation of protein degradation in liver. Diabetes Metab Rev. 1989;5:49–70. - PubMed
-
- Rousset S, et al. UCP2 is a mitochondrial transporter with an unusual very short half-life. FEBS Lett. 2007;581:479–482. - PubMed
-
- Verzijl N, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–39031. - PubMed
-
- Ivan M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science. 2001;292:464–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

