Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis
- PMID: 20699398
- PMCID: PMC2948983
- DOI: 10.1104/pp.110.161109
Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis
Abstract
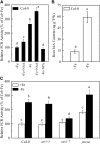
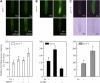
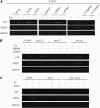
In response to iron (Fe) deficiency, dicots employ a reduction-based mechanism by inducing ferric-chelate reductase (FCR) at the root plasma membrane to enhance Fe uptake. However, the signal pathway leading to FCR induction is still unclear. Here, we found that the Fe-deficiency-induced increase of auxin and nitric oxide (NO) levels in wild-type Arabidopsis (Arabidopsis thaliana) was accompanied by up-regulation of root FCR activity and the expression of the basic helix-loop-helix transcription factor (FIT) and the ferric reduction oxidase 2 (FRO2) genes. This was further stimulated by application of exogenous auxin (α-naphthaleneacetic acid) or NO donor (S-nitrosoglutathione [GSNO]), but suppressed by either polar auxin transport inhibition with 1-naphthylphthalamic acid or NO scavenging with 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, tungstate, or N(ω)-nitro-L-arginine methyl ester hydrochloride. On the other hand, the root FCR activity, NO level, and gene expression of FIT and FRO2 were higher in auxin-overproducing mutant yucca under Fe deficiency, which were sharply restrained by 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide treatment. The opposite response was observed in a basipetal auxin transport impaired mutant aux1-7, which was slightly rescued by exogenous GSNO application. Furthermore, Fe deficiency or α-naphthaleneacetic acid application failed to induce Fe-deficiency responses in noa1 and nial nia2, two mutants with reduced NO synthesis, but root FCR activities in both mutants could be significantly elevated by GSNO. The inability to induce NO burst and FCR activity was further verified in a double mutant yucca noa1 with elevated auxin production and reduced NO accumulation. Therefore, we presented a novel signaling pathway where NO acts downstream of auxin to activate root FCR activity under Fe deficiency in Arabidopsis.
Figures






References
-
- Besson-Bard A, Gravot A, Richaud P, Auroy P, Duc C, Gaymard F, Taconnat L, Renou JP, Pugin A, Wendehenne D. (2009) Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol 149: 1302–1315 - PMC - PubMed
-
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. (2006) ABA induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45: 113–122 - PubMed
-
- Curie C, Briat JF. (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54: 183–206 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

