Endogenous abscisic acid as a key switch for natural variation in flooding-induced shoot elongation
- PMID: 20699400
- PMCID: PMC2949041
- DOI: 10.1104/pp.110.162792
Endogenous abscisic acid as a key switch for natural variation in flooding-induced shoot elongation
Abstract
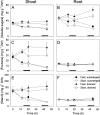
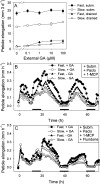
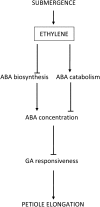
Elongation of leaves and stem is a key trait for survival of terrestrial plants during shallow but prolonged floods that completely submerge the shoot. However, natural floods at different locations vary strongly in duration and depth, and, therefore, populations from these locations are subjected to different selection pressure, leading to intraspecific variation. Here, we identified the signal transduction component that causes response variation in shoot elongation among two accessions of the wetland plant Rumex palustris. These accessions differed 2-fold in petiole elongation rates upon submergence, with fast elongation found in a population from a river floodplain and slow elongation in plants from a lake bank. Fast petiole elongation under water consumes carbohydrates and depends on the (inter)action of the plant hormones ethylene, abscisic acid, and gibberellic acid. We found that carbohydrate levels and dynamics in shoots did not differ between the fast and slow elongating plants, but that the level of ethylene-regulated abscisic acid in petioles, and hence gibberellic acid responsiveness of these petioles explained the difference in shoot elongation upon submergence. Since this is the exact signal transduction level that also explains the variation in flooding-induced shoot elongation among plant species (namely, R. palustris and Rumex acetosa), we suggest that natural selection results in similar modification of regulatory pathways within and between species.
Figures


References
-
- Bailey-Serres J, Voesenek LACJ. (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59: 313–339 - PubMed
-
- Banga M, Bögemann GM, Blom CWPM, Voesenek LACJ. (1997) Flooding resistance of Rumex species strongly depends on their response to ethylene: rapid shoot elongation or foliar senescence. Physiol Plant 99: 415–422
-
- Bartelheimer M, Gowing D, Silvertown J. (2010) Explaining hydrological niches: the decisive role of below-ground competition in two closely related Senecio species. J Ecol 98: 126–136
-
- Benschop JJ, Jackson MB, Guhl K, Vreeburg RAM, Croker SJ, Peeters AJM, Voesenek LACJ. (2005) Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. Plant J 44: 756–768 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

