The role of STEP in Alzheimer's disease
- PMID: 20699650
- PMCID: PMC3230511
- DOI: 10.1523/JNEUROSCI.0157-10.2010
The role of STEP in Alzheimer's disease
Abstract
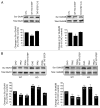
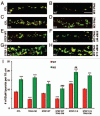
Amyloid beta (Aβ), the putative causative agent in Alzheimer's disease, is known to affect glutamate receptor trafficking. Previous studies have shown that Aβ downregulates the surface expression of N-methyl D-aspartate type glutamate receptors (NMDARs) by the activation of STriatal-Enriched protein tyrosine Phosphatase 61 (STEP₆₁). More recent findings confirm that STEP₆₁ plays an important role in Aβ-induced NMDAR endocytosis. STEP levels are elevated in human AD prefrontal cortex and in the cortex of several AD mouse models. The increase in STEP₆₁ levels and activity contribute to the removal of GluN1/GluN2B receptor complexes from the neuronal surface membranes. The elevation of STEP₆₁ is due to disruption in the normal degradation of STEP₆₁ by the ubiquitin proteasome system. Here, we briefly discuss additional studies in support of our hypothesis that STEP₆₁ contributes to aspects of the pathophysiology in Alzheimer's disease. Exogenous application of Aβ-enriched conditioned medium (7PA2-CM) to wild-type cortical cultures results in a loss of GluN1/GluN2B subunits from neuronal membranes. Abeta-mediated NMDAR internalization does not occur in STEP knock-out cultures, but is rescued by the addition of active TAT-STEP to the cultures prior to Aβ treatment.
Figures
Comment on
-
Abeta-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61.J Neurosci. 2010 Apr 28;30(17):5948-57. doi: 10.1523/JNEUROSCI.0157-10.2010. J Neurosci. 2010. PMID: 20427654 Free PMC article.
Similar articles
-
Abeta-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61.J Neurosci. 2010 Apr 28;30(17):5948-57. doi: 10.1523/JNEUROSCI.0157-10.2010. J Neurosci. 2010. PMID: 20427654 Free PMC article.
-
Reduced levels of the tyrosine phosphatase STEP block β amyloid-mediated GluA1/GluA2 receptor internalization.J Neurochem. 2011 Nov;119(3):664-72. doi: 10.1111/j.1471-4159.2011.07450.x. Epub 2011 Sep 21. J Neurochem. 2011. PMID: 21883219 Free PMC article.
-
NMDA receptors mediate synaptic depression, but not spine loss in the dentate gyrus of adult amyloid Beta (Aβ) overexpressing mice.Acta Neuropathol Commun. 2018 Oct 23;6(1):110. doi: 10.1186/s40478-018-0611-4. Acta Neuropathol Commun. 2018. PMID: 30352630 Free PMC article.
-
Reciprocal disruption of neuronal signaling and Aβ production mediated by extrasynaptic NMDA receptors: a downward spiral.Cell Tissue Res. 2014 May;356(2):279-86. doi: 10.1007/s00441-013-1789-1. Epub 2014 Feb 5. Cell Tissue Res. 2014. PMID: 24496511 Review.
-
Striatal-enriched protein tyrosine phosphatase in Alzheimer's disease.Adv Pharmacol. 2012;64:303-25. doi: 10.1016/B978-0-12-394816-8.00009-X. Adv Pharmacol. 2012. PMID: 22840751 Free PMC article. Review.
Cited by
-
Alzheimer's disease: synapses gone cold.Mol Neurodegener. 2011 Aug 26;6(1):63. doi: 10.1186/1750-1326-6-63. Mol Neurodegener. 2011. PMID: 21871088 Free PMC article.
-
Protein tyrosine phosphatases as potential therapeutic targets.Acta Pharmacol Sin. 2014 Oct;35(10):1227-46. doi: 10.1038/aps.2014.80. Epub 2014 Sep 15. Acta Pharmacol Sin. 2014. PMID: 25220640 Free PMC article. Review.
-
STEP regulation of seizure thresholds in the hippocampus.Epilepsia. 2011 Mar;52(3):497-506. doi: 10.1111/j.1528-1167.2010.02912.x. Epub 2011 Jan 4. Epilepsia. 2011. PMID: 21204826 Free PMC article.
-
Advancement in modeling of Alzheimer's disease: a comprehensive review of preclinical screening platforms.Front Aging Neurosci. 2025 Aug 6;17:1646551. doi: 10.3389/fnagi.2025.1646551. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40842652 Free PMC article. Review.
-
Pushed to extremes: distinct effects of high temperature versus pressure on the structure of STEP.Commun Biol. 2024 Jan 12;7(1):59. doi: 10.1038/s42003-023-05609-0. Commun Biol. 2024. PMID: 38216663 Free PMC article.
References
-
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
