Random mutagenesis screening indicates the absence of a separate H(+)-sensor in the pH-sensitive Kir channels
- PMID: 20699659
- PMCID: PMC3051873
- DOI: 10.4161/chan.4.5.13006
Random mutagenesis screening indicates the absence of a separate H(+)-sensor in the pH-sensitive Kir channels
Abstract
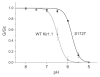
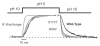
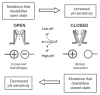
Several inwardly-rectifying (Kir) potassium channels (Kir1.1, Kir4.1 and Kir4.2) are characterised by their sensitivity to inhibition by intracellular H(+) within the physiological range. The mechanism by which these channels are regulated by intracellular pH has been the subject of intense scrutiny for over a decade, yet the molecular identity of the titratable pH-sensor remains elusive. In this study we have taken advantage of the acidic intracellular environment of S. cerevisiae and used a K(+) -auxotrophic strain to screen for mutants of Kir1.1 with impaired pH-sensitivity. In addition to the previously identified K80M mutation, this unbiased screening approach identified a novel mutation (S172T) in the second transmembrane domain (TM2) that also produces a marked reduction in pH-sensitivity through destabilization of the closed-state. However, despite this extensive mutagenic approach, no mutations could be identified which removed channel pH-sensitivity or which were likely to act as a separate H(+) -sensor unique to the pH-sensitive Kir channels. In order to explain these results we propose a model in which the pH-sensing mechanism is part of an intrinsic gating mechanism common to all Kir channels, not just the pH-sensitive Kir channels. In this model, mutations which disrupt this pH-sensor would result in an increase, not reduction, in pH-sensitivity. This has major implications for any future studies of Kir channel pH-sensitivity and explains why formal identification of these pH-sensing residues still represents a major challenge.
Figures



References
-
- Bichet D, Haass FA, Jan LY. Merging functional studies with structures of inward-rectifier K+ channels. Nat Rev Neurosci. 2003;4:957–967. - PubMed
-
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function and physiological roles. Physiol Rev. 2010;90:291–366. - PubMed
-
- Schulte U, Fakler B. Gating of inward-rectifier K+ channels by intracellular pH. Eur J Biochem. 2000;267:5837–5841. - PubMed
-
- Hebert SC. Bartter syndrome. Curr Opin Nephrol Hypertens. 2003;12:527–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
