POD nanoparticles expressing GDNF provide structural and functional rescue of light-induced retinal degeneration in an adult mouse
- PMID: 20700110
- PMCID: PMC2990513
- DOI: 10.1038/mt.2010.167
POD nanoparticles expressing GDNF provide structural and functional rescue of light-induced retinal degeneration in an adult mouse
Abstract
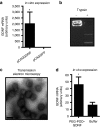
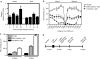
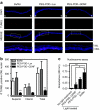
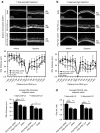
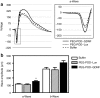
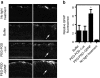
Peptide for ocular delivery (POD) is a novel cationic cell-penetrating peptide (CPP) which, when conjugated with polyethylene glycol (PEG-POD), can deliver plasmid DNA to the retinal pigment epithelium (RPE) of adult murine retina. PEG-POD nanoparticles containing an expression cassette for glial cell line-derived neurotrophic factor (PEG-POD~GDNF) were investigated for their ability to inhibit light-induced photoreceptor apoptosis. PEG-POD~GDNF, control nanoparticles, or buffer were injected into the subretinal space of adult murine retina and retinal degeneration induced by blue light. Animals injected with PEG-POD~GDNF showed a significant reduction (3.9-7.7 fold) in apoptosis relative to control-injected animals. The thickness of the outer nuclear layer (ONL) of the superior retina of PEG-POD~GDNF-injected eyes was significantly greater (23.6-39.3%) than control-injected retina 14 days post-light treatment. PEG-POD~GDNF-injected eyes showed a 27-39% greater functional response relative to controls, as measured by electroretinogram (ERG) 7 days post-light treatment. This is one of only two studies demonstrating histological and functional rescue of a mouse model of retinal degeneration following nonviral administration of a transgene into adult retina. Although rescue is short lived for clinical application, this study represents an important step in the development of nonviral gene therapy for retinal diseases.
Figures
Similar articles
-
Ex vivo gene therapy using intravitreal injection of GDNF-secreting mouse embryonic stem cells in a rat model of retinal degeneration.Mol Vis. 2009 May 13;15:962-73. Mol Vis. 2009. PMID: 19461934 Free PMC article.
-
Constitutive and AP20187-induced Ret activation in photoreceptors does not protect from light-induced damage.Invest Ophthalmol Vis Sci. 2007 Nov;48(11):5199-206. doi: 10.1167/iovs.07-0140. Invest Ophthalmol Vis Sci. 2007. PMID: 17962474
-
Expression of neurturin, glial cell line-derived neurotrophic factor, and their receptor components in light-induced retinal degeneration.Invest Ophthalmol Vis Sci. 2004 Apr;45(4):1240-6. doi: 10.1167/iovs.03-1122. Invest Ophthalmol Vis Sci. 2004. PMID: 15037593
-
Delayed loss of cone and remaining rod photoreceptor cells due to impairment of choroidal circulation after acute light exposure in rats.Invest Ophthalmol Vis Sci. 2007 Apr;48(4):1864-72. doi: 10.1167/iovs.06-1065. Invest Ophthalmol Vis Sci. 2007. PMID: 17389522
-
Gene Therapy for Retinal Degenerative Diseases: Progress, Challenges, and Future Directions.Invest Ophthalmol Vis Sci. 2023 Jun 1;64(7):39. doi: 10.1167/iovs.64.7.39. Invest Ophthalmol Vis Sci. 2023. PMID: 37389545 Free PMC article. Review.
Cited by
-
The utility and risks of therapeutic nanotechnology in the retina.Ther Adv Ophthalmol. 2021 Mar 22;13:25158414211003381. doi: 10.1177/25158414211003381. eCollection 2021 Jan-Dec. Ther Adv Ophthalmol. 2021. PMID: 33817552 Free PMC article. Review.
-
Effective In Vivo Topical Delivery of siRNA and Gene Silencing in Intact Corneal Epithelium Using a Modified Cell-Penetrating Peptide.Mol Ther Nucleic Acids. 2019 Sep 6;17:891-906. doi: 10.1016/j.omtn.2019.07.017. Epub 2019 Aug 1. Mol Ther Nucleic Acids. 2019. PMID: 31476668 Free PMC article.
-
Promising Approach in the Treatment of Glaucoma Using Nanotechnology and Nanomedicine-Based Systems.Molecules. 2019 Oct 22;24(20):3805. doi: 10.3390/molecules24203805. Molecules. 2019. PMID: 31652593 Free PMC article. Review.
-
Loss of daylight vision in retinal degeneration: are oxidative stress and metabolic dysregulation to blame?J Biol Chem. 2012 Jan 13;287(3):1642-8. doi: 10.1074/jbc.R111.304428. Epub 2011 Nov 10. J Biol Chem. 2012. PMID: 22074929 Free PMC article. Review.
-
Stem cell therapy in retinal diseases.Neural Regen Res. 2023 Jul;18(7):1478-1485. doi: 10.4103/1673-5374.361537. Neural Regen Res. 2023. PMID: 36571345 Free PMC article. Review.
References
-
- Klein R, Klein BE., and, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. - PubMed
-
- Lindgren M, Hällbrink M, Prochiantz A., and, Langel U. Cell-penetrating peptides. Trends Pharmacol Sci. 2000;21:99–103. - PubMed
-
- Cashman SM, Morris DJ., and, Kumar-Singh R. Evidence of protein transduction but not intercellular transport by proteins fused to HIV tat in retinal cell culture and in vivo. Mol Ther. 2003;8:130–142. - PubMed
-
- Cashman SM, Sadowski SL, Morris DJ, Frederick J., and, Kumar-Singh R. Intercellular trafficking of adenovirus-delivered HSV VP22 from the retinal pigment epithelium to the photoreceptors–implications for gene therapy. Mol Ther. 2002;6:813–823. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

