Selectivity of oncolytic viral replication prevents antiviral immune response and toxicity, but does not improve antitumoral immunity
- PMID: 20700112
- PMCID: PMC2990511
- DOI: 10.1038/mt.2010.163
Selectivity of oncolytic viral replication prevents antiviral immune response and toxicity, but does not improve antitumoral immunity
Abstract
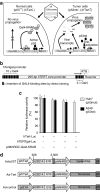
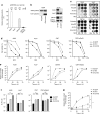
Oncolytic infection elicits antitumoral immunity, but the impact of tumor-selective replication on the balance between antiviral and antitumoral immune responses has not yet been investigated. To address this question, we constructed the highly tumor-selective adenovirus Ad-p53T whose replication in target tumor cells is governed by aberrant telomerase activity and transcriptional p53 dysfunction. Telomerase-dependent or nonselective adenoviruses were constructed as isogenic controls. Following infection of mice with the nonselective adenovirus, viral DNA and mRNA levels correlated with strong stimulation of innate immune response genes and severe liver toxicity, whereas telomerase-/p53-specific replication did not trigger innate immunity and prevented liver damage. Compared to telomerase-dependent or unselective viral replication, telomerase-/p53-specific virotherapy significantly decreased antiviral CD8-specific immune responses and antiviral cytotoxicity in vivo. Consistent with our hypothesis, telomerase-selective replication led to intermediate results in these experiments. Remarkably, all viruses efficiently lysed tumors and induced a therapeutically effective tumor-directed CD8 cytotoxicity. In immunocompetent mice with extended lung metastases burden, treatment of subcutaneous primary tumors with Ad-p53T significantly prolonged survival by inhibition of lung metastases, whereas unselective viral replication resulted in death by liver failure. In summary, the degree of tumor selectivity of viral replication marginally influences antitumoral immune responses, but is a major determinant of antivector immunity and systemic toxicity.
Figures




Similar articles
-
Telomerase-specific replication-selective virotherapy for human cancer.Clin Cancer Res. 2004 Jan 1;10(1 Pt 1):285-92. doi: 10.1158/1078-0432.ccr-1075-3. Clin Cancer Res. 2004. PMID: 14734481
-
Functional interactions of antiapoptotic proteins and tumor necrosis factor in the context of a replication-competent adenovirus.Gene Ther. 2005 Sep;12(17):1333-46. doi: 10.1038/sj.gt.3302555. Gene Ther. 2005. PMID: 15920462
-
Antitumoral immune response by recruitment and expansion of dendritic cells in tumors infected with telomerase-dependent oncolytic viruses.Cancer Res. 2009 Feb 15;69(4):1448-58. doi: 10.1158/0008-5472.CAN-08-1160. Epub 2009 Feb 3. Cancer Res. 2009. PMID: 19190348
-
Telomerase-specific oncolytic virotherapy for human gastrointestinal cancer.Expert Rev Anticancer Ther. 2011 Apr;11(4):525-32. doi: 10.1586/era.10.200. Expert Rev Anticancer Ther. 2011. PMID: 21504319 Review.
-
Telomerase-specific oncolytic virotherapy for human cancer with the hTERT promoter.Curr Cancer Drug Targets. 2007 Mar;7(2):191-201. doi: 10.2174/156800907780058835. Curr Cancer Drug Targets. 2007. PMID: 17346111 Review.
Cited by
-
Oncolytic viruses as therapeutic cancer vaccines.Mol Cancer. 2013 Sep 11;12(1):103. doi: 10.1186/1476-4598-12-103. Mol Cancer. 2013. PMID: 24020520 Free PMC article. Review.
-
Ad3-hTERT-E1A, a fully serotype 3 oncolytic adenovirus, in patients with chemotherapy refractory cancer.Mol Ther. 2012 Sep;20(9):1821-1830. doi: 10.1038/mt.2012.115. Epub 2012 Aug 7. Mol Ther. 2012. PMID: 22871667 Free PMC article.
-
Combined therapy with CTL cells and oncolytic adenovirus expressing IL-15-induced enhanced antitumor activity.Tumour Biol. 2015 Jun;36(6):4535-43. doi: 10.1007/s13277-015-3098-7. Epub 2015 Jan 28. Tumour Biol. 2015. PMID: 25627006
-
Meganuclease-mediated virus self-cleavage facilitates tumor-specific virus replication.Mol Ther. 2013 Sep;21(9):1738-48. doi: 10.1038/mt.2013.117. Epub 2013 Jun 11. Mol Ther. 2013. PMID: 23752311 Free PMC article.
-
Showing the Way: Oncolytic Adenoviruses as Chaperones of Immunostimulatory Adjuncts.Biomedicines. 2016 Sep 19;4(3):23. doi: 10.3390/biomedicines4030023. Biomedicines. 2016. PMID: 28536390 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

