Tumor necrosis factor α primes cerebral endothelial cells for erythropoietin-induced angiogenesis
- PMID: 20700128
- PMCID: PMC3049518
- DOI: 10.1038/jcbfm.2010.138
Tumor necrosis factor α primes cerebral endothelial cells for erythropoietin-induced angiogenesis
Erratum in
- J Cereb Blood Flow Metab. 2011 May;31(5):1334. Francisco, Moniche Ãlvarez [corrected to Francisco, Moniche-Alvarez]
Abstract
Erythropoietin (EPO) enhances angiogenesis in the ischemic brain. Stroke induces secretion of tumor necrosis factor α (TNF-α). We investigated the effect of TNF-α on EPO-induced in vitro angiogenesis in cerebral endothelial cells. Using a capillary-like tubular formation assay, we found that transient incubation of primary rat cerebral microvascular endothelial cells (RECs) with TNF-α substantially upregulated EPO receptor (EPOR) expression and addition of EPO into TNF-α-treated RECs significantly augmented the capillary-like tube formation. Blockage of TNF receptor 1 (TNFR1) suppressed TNF-α-upregulated EPOR expression and abolished EPO-induced tube formation. Attenuation of endogenous EPOR with small interfering RNA (siRNA) also inhibited EPO-enhanced tube formation. Treatment of RECs with EPO activated nuclear factor-kappa B (NF-κB) and Akt. Incubation of the TNF-α-treated endothelial cells with EPO activated vascular endothelial growth factor (VEGF), VEGF receptor 2 (VEGFR2), angiopoietin 1 (Ang1), and Tie2. Blockage of VEGFR2 and Tie2 resulted in reduction of EPO-augmented tube formation. These data indicate that interaction of TNF-α with TNFR1 sensitizes cerebral endothelial cells for EPO-induced angiogenesis by upregulation of EPOR, which amplifies the effect of EPO on activation of the VEGF/VEGFR2 and Ang1/Tie2 pathways. Our results provide the evidence for crosslink between TNF and EPOR to coordinate the onset of angiogenesis in cerebral endothelial cells.
Figures


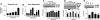
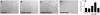
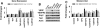
Similar articles
-
Erythropoietin attenuates cardiac dysfunction by increasing myocardial angiogenesis and inhibiting interstitial fibrosis in diabetic rats.Cardiovasc Diabetol. 2012 Sep 7;11:105. doi: 10.1186/1475-2840-11-105. Cardiovasc Diabetol. 2012. PMID: 22954171 Free PMC article.
-
VEGFA activates erythropoietin receptor and enhances VEGFR2-mediated pathological angiogenesis.Am J Pathol. 2014 Apr;184(4):1230-1239. doi: 10.1016/j.ajpath.2013.12.023. Epub 2014 Mar 12. Am J Pathol. 2014. PMID: 24630601 Free PMC article.
-
Erythropoietin-dependent autocrine secretion of tumor necrosis factor-alpha in hematopoietic cells modulates proliferation via MAP kinase--ERK-1/2 and does not require tyrosine docking sites in the EPO receptor.Exp Cell Res. 2004 Aug 1;298(1):155-66. doi: 10.1016/j.yexcr.2004.04.009. Exp Cell Res. 2004. PMID: 15242770
-
Mystery Story about Erythropoietin (Epo) and Erythropoietin Receptor (EpoR) are Disguised?Hepatogastroenterology. 2015 May;62(139):585-9. Hepatogastroenterology. 2015. PMID: 26897933 Review.
-
New avenues of exploration for erythropoietin.JAMA. 2005 Jan 5;293(1):90-5. doi: 10.1001/jama.293.1.90. JAMA. 2005. PMID: 15632341 Free PMC article. Review.
Cited by
-
Stem cell-paved biobridges facilitate stem transplant and host brain cell interactions for stroke therapy.Brain Res. 2015 Oct 14;1623:160-5. doi: 10.1016/j.brainres.2015.03.007. Epub 2015 Mar 11. Brain Res. 2015. PMID: 25770817 Free PMC article. Review.
-
The receptor that tames the innate immune response.Mol Med. 2012 May 9;18(1):486-96. doi: 10.2119/molmed.2011.00414. Mol Med. 2012. PMID: 22183892 Free PMC article. Review.
-
Dose-dependent neurorestorative effects of delayed treatment of traumatic brain injury with recombinant human erythropoietin in rats.J Neurosurg. 2011 Sep;115(3):550-60. doi: 10.3171/2011.3.JNS101721. Epub 2011 Apr 15. J Neurosurg. 2011. PMID: 21495821 Free PMC article.
-
Infections in the Developing Brain: The Role of the Neuro-Immune Axis.Front Neurol. 2022 Feb 17;13:805786. doi: 10.3389/fneur.2022.805786. eCollection 2022. Front Neurol. 2022. PMID: 35250814 Free PMC article. Review.
-
Cellular and molecular mechanisms in vascular repair after traumatic brain injury: a narrative review.Burns Trauma. 2023 Sep 4;11:tkad033. doi: 10.1093/burnst/tkad033. eCollection 2023. Burns Trauma. 2023. PMID: 37675267 Free PMC article. Review.
References
-
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. - PubMed
-
- Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–494. - PubMed
-
- Carlini RG, Reyes AA, Rothstein M. Recombinant human erythropoietin stimulates angiogenesis in vitro. Kidney Int. 1995;47:740–745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

