α-MSH: a potential neuroprotective and immunomodulatory agent for the treatment of stroke
- PMID: 20700130
- PMCID: PMC3049515
- DOI: 10.1038/jcbfm.2010.130
α-MSH: a potential neuroprotective and immunomodulatory agent for the treatment of stroke
Abstract
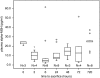
Alpha-melanocyte-stimulating hormone (MSH) is a neuropeptide with profound immunomodulatory properties; we evaluated the effects of α-MSH on stroke outcome and its ability to modulate the postischemic immune response. In Lewis rats subjected to 3 hours of middle cerebral artery occlusion (MCAO), plasma concentrations of α-MSH rapidly decreased and returned to baseline over the course of days. Exogenous administration of α-MSH (100 or 500 μg/kg) improved 24 hour outcome in animals subjected to 2 hours MCAO; α-MSH 500 μg/kg also decreased infarct volume at this time point. Both doses of α-MSH were ineffective in improving outcome or decreasing infarct volume in animals subjected to 3 hours MCAO. The splenocyte response to phytohemagglutin in animals treated with α-MSH was attenuated at 24 hours after MCAO. At 1 month after MCAO, treatment with α-MSH 500 μg/kg at the time of stoke was associated with a decrease in TH1 response to myelin basic protein (MBP) in animals subjected to 2 hours MCAO, although treatment was not associated with improved outcome at this time point. Given the early benefits of α-MSH treatment and its effect on immunologic outcome, further studies to evaluate the utility of α-MSH for the treatment of cerebral ischemia are warranted.
Figures


Similar articles
-
Reduction of ischemic stroke in rat brain by alpha melanocyte stimulating hormone.Neuropeptides. 2008 Jun;42(3):331-8. doi: 10.1016/j.npep.2008.01.004. Epub 2008 Mar 24. Neuropeptides. 2008. PMID: 18359516
-
Alpha-MSH promotes spontaneous post-ischemic pneumonia in mice via melanocortin-receptor-1.Exp Neurol. 2008 Apr;210(2):731-9. doi: 10.1016/j.expneurol.2008.01.006. Epub 2008 Jan 19. Exp Neurol. 2008. PMID: 18304533
-
Plasma α-melanocyte stimulating hormone predicts outcome in ischemic stroke.Stroke. 2011 Dec;42(12):3415-20. doi: 10.1161/STROKEAHA.111.627331. Epub 2011 Sep 29. Stroke. 2011. PMID: 21960572 Free PMC article.
-
Adoptive transfer of myelin basic protein-tolerized splenocytes to naive animals reduces infarct size: a role for lymphocytes in ischemic brain injury?Stroke. 2003 Jul;34(7):1809-15. doi: 10.1161/01.STR.0000078308.77727.EA. Epub 2003 Jun 5. Stroke. 2003. PMID: 12791945
-
Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window.Stroke. 2001 Feb;32(2):553-60. doi: 10.1161/01.str.32.2.553. Stroke. 2001. PMID: 11157196
Cited by
-
The Skin-Brain Axis: From UV and Pigmentation to Behaviour Modulation.Int J Mol Sci. 2024 Jun 4;25(11):6199. doi: 10.3390/ijms25116199. Int J Mol Sci. 2024. PMID: 38892387 Free PMC article. Review.
-
Method parameters' impact on mortality and variability in rat stroke experiments: a meta-analysis.BMC Neurosci. 2013 Apr 1;14:41. doi: 10.1186/1471-2202-14-41. BMC Neurosci. 2013. PMID: 23548160 Free PMC article.
-
A feasibility and safety study of afamelanotide in acute stroke patients - an open label, proof of concept, phase iia clinical trial.BMC Neurol. 2023 Jul 26;23(1):281. doi: 10.1186/s12883-023-03338-9. BMC Neurol. 2023. PMID: 37496004 Free PMC article. Clinical Trial.
-
α-Melanocyte-Stimulating Hormone Attenuates Neovascularization by Inducing Nitric Oxide Deficiency via MC-Rs/PKA/NF-κB Signaling.Int J Mol Sci. 2018 Nov 30;19(12):3823. doi: 10.3390/ijms19123823. Int J Mol Sci. 2018. PMID: 30513637 Free PMC article.
-
Single administration of tripeptide α-MSH(11-13) attenuates brain damage by reduced inflammation and apoptosis after experimental traumatic brain injury in mice.PLoS One. 2013 Aug 5;8(8):e71056. doi: 10.1371/journal.pone.0071056. Print 2013. PLoS One. 2013. PMID: 23940690 Free PMC article.
References
-
- Becker K, Kindrick D, McCarron R, Hallenbeck J, Winn R. Adoptive transfer of myelin basic protein-tolerized splenocytes to naive animals reduces infarct size: a role for lymphocytes in ischemic brain injury. Stroke. 2003;34:1809–1815. - PubMed
-
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

