New functions of aminoacyl-tRNA synthetases beyond translation
- PMID: 20700144
- PMCID: PMC3042954
- DOI: 10.1038/nrm2956
New functions of aminoacyl-tRNA synthetases beyond translation
Abstract
Over the course of evolution, eukaryotic aminoacyl-tRNA synthetases (aaRSs) progressively incorporated domains and motifs that have no essential connection to aminoacylation reactions. Their accretive addition to virtually all aaRSs correlates with the progressive evolution and complexity of eukaryotes. Based on recent experimental findings focused on a few of these additions and analysis of the aaRS proteome, we propose that they are markers for aaRS-associated functions beyond translation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
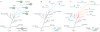

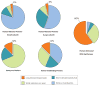
References
-
- Carter CW., Jr Cognition, mechanism, and evolutionary relationships in aminoacyl-tRNA synthetases. Annu Rev Biochem. 1993;62:715–48. - PubMed
-
- Rodin SN, Ohno S. Two types of aminoacyl-tRNA synthetases could be originally encoded by complementary strands of the same nucleic acid. Orig Life Evol Biosph. 1995;25:565–89. - PubMed
-
- Pham Y, et al. A minimal TrpRS catalytic domain supports sense/antisense ancestry of class I and II aminoacyl-tRNA synthetases. Mol Cell. 2007;25:851–62. - PubMed
-
- Ribas de Pouplana L, Schimmel P. Two classes of tRNA synthetases suggested by sterically compatible dockings on tRNA acceptor stem. Cell. 2001;104:191–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

