Imaging and recording subventricular zone progenitor cells in live tissue of postnatal mice
- PMID: 20700392
- PMCID: PMC2918349
- DOI: 10.3389/fnins.2010.00043
Imaging and recording subventricular zone progenitor cells in live tissue of postnatal mice
Abstract
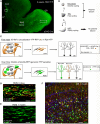
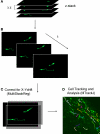
The subventricular zone (SVZ) is one of two regions where neurogenesis persists in the postnatal brain. The SVZ, located along the lateral ventricle, is the largest neurogenic zone in the brain that contains multiple cell populations including astrocyte-like cells and neuroblasts. Neuroblasts migrate in chains to the olfactory bulb where they differentiate into interneurons. Here, we discuss the experimental approaches to record the electrophysiology of these cells and image their migration and calcium activity in acute slices. Although these techniques were in place for studying glial cells and neurons in mature networks, the SVZ raises new challenges due to the unique properties of SVZ cells, the cellular diversity, and the architecture of the region. We emphasize different methods, such as the use of transgenic mice and in vivo electroporation that permit identification of the different SVZ cell populations for patch clamp recording or imaging. Electroporation also permits genetic labeling of cells using fluorescent reporter mice and modification of the system using either RNA interference technology or floxed mice. In this review, we aim to provide conceptual and technical details of the approaches to perform electrophysiological and imaging studies of SVZ cells.
Keywords: calcium imaging; electroporation; migration; neuroblasts; neurogenesis; proliferation; stem cells; transgenic mice.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

