Design, synthesis, and analysis of minor groove binder pyrrolepolyamide-2'-deoxyguanosine hybrids
- PMID: 20700414
- PMCID: PMC2911594
- DOI: 10.4061/2010/235240
Design, synthesis, and analysis of minor groove binder pyrrolepolyamide-2'-deoxyguanosine hybrids
Abstract
Pyrrolepolyamide-2'-deoxyguanosine hybrids (Hybrid 2 and Hybrid 3) incorporating the 3-aminopropionyl or 3-aminopropyl linker were designed and synthesized on the basis of previously reported results of a pyrrolepolyamide-adenosine hybrid (Hybrid 1). Evaluation of the DNA binding sequence selectivity of pyrrolepolyamide-2'-deoxyguanosine hybrids was performed by CD spectral and T(m) analyses. It was shown that Hybrid 3 possessed greater binding specificity than distamycin A, Hybrid 1 and Hybrid 2.
Figures


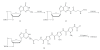



Similar articles
-
[Design, synthesis and evaluation of polyamide-nucleoside hybrids and oligonucleotides conjugated hybrid as a novel gene expression control compound].Yakugaku Zasshi. 2010 Mar;130(3):355-75. doi: 10.1248/yakushi.130.355. Yakugaku Zasshi. 2010. PMID: 20190521 Review. Japanese.
-
Design, synthesis and analysis of a pyrrolepolyamide-nucleoside hybrid.Nucleic Acids Symp Ser (Oxf). 2004;(48):55-6. doi: 10.1093/nass/48.1.55. Nucleic Acids Symp Ser (Oxf). 2004. PMID: 17150475
-
Design, synthesis and evaluation of oligomer conjugated MGBpolyamide-nucleoside hybrid as a novel gene expression control compound.Nucleic Acids Symp Ser (Oxf). 2005;(49):327-8. doi: 10.1093/nass/49.1.327. Nucleic Acids Symp Ser (Oxf). 2005. PMID: 17150766
-
Synthesis and evaluation of 2-pyrrolepolyamide-2'-deoxyguanosine 5'-phosphate.Nucleic Acids Symp Ser (Oxf). 2007;(51):263-4. doi: 10.1093/nass/nrm132. Nucleic Acids Symp Ser (Oxf). 2007. PMID: 18029687
-
Distamycin A as stem of DNA minor groove alkylating agents.Curr Top Med Chem. 2004;4(2):231-9. doi: 10.2174/1568026043451474. Curr Top Med Chem. 2004. PMID: 14754456 Review.
Cited by
-
Synthesis and Evaluation of MGB Polyamide-Oligonucleotide Conjugates as Gene Expression Control Compounds.J Nucleic Acids. 2023 Mar 14;2023:2447998. doi: 10.1155/2023/2447998. eCollection 2023. J Nucleic Acids. 2023. PMID: 36960406 Free PMC article.
References
-
- Cook PD. A brief history, status, and perspective of modified oligonucleotides for chemotherapeutic applications. In: Beaucage SL, Bergstrom DE, Glick GD, Jones RA, editors. Current Protocols in Nucleic Acid Chemistry. New York, NY, USA: John Wiley & Sons; 2000. pp. 4.1.1–4.1.17. - PubMed
-
- Huang L, Quada JC, Jr., Lown JW. Functional models of the antitumor antibiotic bleomycin. Current Medicinal Chemistry. 1995;2(1):543–560.
-
- Baird EE, Dervan PB. Solid phase synthesis of polyamides containing imidazole and pyrrole amino acids. Journal of the American Chemical Society. 1996;118(26):6141–6146.
-
- Wang Y, Gupta R, Huang L, Luo W, Lown JW. Design, synthesis, cytotoxic properties and preliminary DNA sequencing evaluation of CPI-N-methylpyrrole hybrids. Enhancing effect of a trans double bond linker and role of the terminal amide functionality on cytotoxic potency. Anti-Cancer Drug Design. 1996;11(1):15–34. - PubMed
LinkOut - more resources
Full Text Sources

