A developmental timing switch promotes axon outgrowth independent of known guidance receptors
- PMID: 20700435
- PMCID: PMC2916846
- DOI: 10.1371/journal.pgen.1001054
A developmental timing switch promotes axon outgrowth independent of known guidance receptors
Abstract
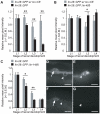
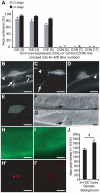
To form functional neuronal connections, axon outgrowth and guidance must be tightly regulated across space as well as time. While a number of genes and pathways have been shown to control spatial features of axon development, very little is known about the in vivo mechanisms that direct the timing of axon initiation and elongation. The Caenorhabditis elegans hermaphrodite specific motor neurons (HSNs) extend a single axon ventrally and then anteriorly during the L4 larval stage. Here we show the lin-4 microRNA promotes HSN axon initiation after cell cycle withdrawal. Axons fail to form in lin-4 mutants, while they grow prematurely in lin-4-overexpressing animals. lin-4 is required to down-regulate two inhibitors of HSN differentiation--the transcriptional regulator LIN-14 and the "stemness" factor LIN-28--and it likely does so through a cell-autonomous mechanism. This developmental switch depends neither on the UNC-40/DCC and SAX-3/Robo receptors nor on the direction of axon growth, demonstrating that it acts independently of ventral guidance signals to control the timing of HSN axon elongation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) function interdependently to promote axon guidance by regulating the MIG-2 GTPase.PLoS Genet. 2015 Apr 15;11(4):e1005185. doi: 10.1371/journal.pgen.1005185. eCollection 2015 Apr. PLoS Genet. 2015. PMID: 25876065 Free PMC article.
-
The lin-4 microRNA targets the LIN-14 transcription factor to inhibit netrin-mediated axon attraction.Sci Signal. 2012 Jun 12;5(228):ra43. doi: 10.1126/scisignal.2002437. Sci Signal. 2012. PMID: 22692424 Free PMC article.
-
The POU transcription factor UNC-86 controls the timing and ventral guidance of Caenorhabditis elegans axon growth.Dev Dyn. 2011 Jul;240(7):1815-25. doi: 10.1002/dvdy.22667. Epub 2011 Jun 8. Dev Dyn. 2011. PMID: 21656875 Free PMC article.
-
Localization mechanisms of the axon guidance molecule UNC-6/Netrin and its receptors, UNC-5 and UNC-40, in Caenorhabditis elegans.Dev Growth Differ. 2012 Apr;54(3):390-7. doi: 10.1111/j.1440-169X.2012.01349.x. Dev Growth Differ. 2012. PMID: 22524608 Review.
-
Netrin, Slit and Wnt receptors allow axons to choose the axis of migration.Dev Biol. 2008 Nov 15;323(2):143-51. doi: 10.1016/j.ydbio.2008.08.027. Epub 2008 Sep 5. Dev Biol. 2008. PMID: 18801355 Review.
Cited by
-
A novel mechanism of LIN-28 regulation of let-7 microRNA expression revealed by in vivo HITS-CLIP in C. elegans.RNA. 2015 May;21(5):985-96. doi: 10.1261/rna.045542.114. Epub 2015 Mar 24. RNA. 2015. PMID: 25805859 Free PMC article.
-
Recent Molecular Genetic Explorations of Caenorhabditis elegans MicroRNAs.Genetics. 2018 Jul;209(3):651-673. doi: 10.1534/genetics.118.300291. Genetics. 2018. PMID: 29967059 Free PMC article.
-
Gata3 is a critical regulator of cochlear wiring.J Neurosci. 2013 Feb 20;33(8):3679-91. doi: 10.1523/JNEUROSCI.4703-12.2013. J Neurosci. 2013. PMID: 23426694 Free PMC article.
-
Persistent Lin28 Expression Impairs Neurite Outgrowth and Cognitive Function in the Developing Mouse Neocortex.Mol Neurobiol. 2019 May;56(5):3780-3795. doi: 10.1007/s12035-018-1297-0. Epub 2018 Sep 10. Mol Neurobiol. 2019. PMID: 30203263
-
MicroRNAs: Not "Fine-Tuners" but Key Regulators of Neuronal Development and Function.Front Neurol. 2015 Nov 24;6:245. doi: 10.3389/fneur.2015.00245. eCollection 2015. Front Neurol. 2015. PMID: 26635721 Free PMC article. Review.
References
-
- Polleux F, Ince-Dunn G, Ghosh A. Transcriptional regulation of vertebrate axon guidance and synapse formation. Nat Rev Neurosci. 2007;8:331–340. - PubMed
-
- Kennedy TE. Cellular mechanisms of netrin function: long-range and short-range actions. Biochem Cell Biol. 2000;78:569–575. - PubMed
-
- Butler SJ, Tear G. Getting axons onto the right path: the role of transcription factors in axon guidance. Development. 2007;134:439–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

