RNAi screening implicates a SKN-1-dependent transcriptional response in stress resistance and longevity deriving from translation inhibition
- PMID: 20700440
- PMCID: PMC2916858
- DOI: 10.1371/journal.pgen.1001048
RNAi screening implicates a SKN-1-dependent transcriptional response in stress resistance and longevity deriving from translation inhibition
Abstract
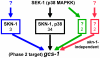
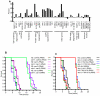
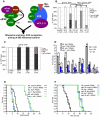
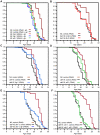
Caenorhabditis elegans SKN-1 (ortholog of mammalian Nrf1/2/3) is critical for oxidative stress resistance and promotes longevity under reduced insulin/IGF-1-like signaling (IIS), dietary restriction (DR), and normal conditions. SKN-1 inducibly activates genes involved in detoxification, protein homeostasis, and other functions in response to stress. Here we used genome-scale RNA interference (RNAi) screening to identify mechanisms that prevent inappropriate SKN-1 target gene expression under non-stressed conditions. We identified 41 genes for which knockdown leads to activation of a SKN-1 target gene (gcs-1) through skn-1-dependent or other mechanisms. These genes correspond to multiple cellular processes, including mRNA translation. Inhibition of translation is known to increase longevity and stress resistance and may be important for DR-induced lifespan extension. One model postulates that these effects derive from reduced energy needs, but various observations suggest that specific longevity pathways are involved. Here we show that translation initiation factor RNAi robustly induces SKN-1 target gene transcription and confers skn-1-dependent oxidative stress resistance. The accompanying increases in longevity are mediated largely through the activities of SKN-1 and the transcription factor DAF-16 (FOXO), which is required for longevity that derives from reduced IIS. Our results indicate that the SKN-1 detoxification gene network monitors various metabolic and regulatory processes. Interference with one of these processes, translation initiation, leads to a transcriptional response whereby SKN-1 promotes stress resistance and functions together with DAF-16 to extend lifespan. This stress response may be beneficial for coping with situations that are associated with reduced protein synthesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans.Cell. 2008 Mar 21;132(6):1025-38. doi: 10.1016/j.cell.2008.01.030. Cell. 2008. PMID: 18358814 Free PMC article.
-
The SKN-1/Nrf2 transcription factor can protect against oxidative stress and increase lifespan in C. elegans by distinct mechanisms.Aging Cell. 2017 Oct;16(5):1191-1194. doi: 10.1111/acel.12627. Epub 2017 Jun 14. Aging Cell. 2017. PMID: 28612944 Free PMC article.
-
Temporal requirements of SKN-1/NRF as a regulator of lifespan and proteostasis in Caenorhabditis elegans.PLoS One. 2021 Jul 1;16(7):e0243522. doi: 10.1371/journal.pone.0243522. eCollection 2021. PLoS One. 2021. PMID: 34197476 Free PMC article.
-
SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans.Free Radic Biol Med. 2015 Nov;88(Pt B):290-301. doi: 10.1016/j.freeradbiomed.2015.06.008. Epub 2015 Aug 5. Free Radic Biol Med. 2015. PMID: 26232625 Free PMC article. Review.
-
Unique structure and regulation of the nematode detoxification gene regulator, SKN-1: implications to understanding and controlling drug resistance.Drug Metab Rev. 2012 Aug;44(3):209-23. doi: 10.3109/03602532.2012.684799. Epub 2012 Jun 4. Drug Metab Rev. 2012. PMID: 22656429 Free PMC article. Review.
Cited by
-
Acetyl-CoA Metabolism and Histone Acetylation in the Regulation of Aging and Lifespan.Antioxidants (Basel). 2021 Apr 8;10(4):572. doi: 10.3390/antiox10040572. Antioxidants (Basel). 2021. PMID: 33917812 Free PMC article. Review.
-
FOXO target gene CTDSP2 regulates cell cycle progression through Ras and p21(Cip1/Waf1).Biochem J. 2015 Jul 15;469(2):289-98. doi: 10.1042/BJ20140831. Epub 2015 May 20. Biochem J. 2015. PMID: 25990325 Free PMC article.
-
The ubiquitin proteasome system in Caenorhabditis elegans and its regulation.Redox Biol. 2014 Jan 18;2:333-47. doi: 10.1016/j.redox.2014.01.007. eCollection 2014. Redox Biol. 2014. PMID: 24563851 Free PMC article. Review.
-
D-Glucosamine supplementation extends life span of nematodes and of ageing mice.Nat Commun. 2014 Apr 8;5:3563. doi: 10.1038/ncomms4563. Nat Commun. 2014. PMID: 24714520 Free PMC article.
-
Neuronal protein with tau-like repeats (PTL-1) regulates intestinal SKN-1 nuclear accumulation in response to oxidative stress.Aging Cell. 2015 Feb;14(1):148-51. doi: 10.1111/acel.12285. Epub 2014 Nov 14. Aging Cell. 2015. PMID: 25399685 Free PMC article.
References
-
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. - PubMed
-
- Lithgow GJ, Walker GA. Stress resistance as a determinate of C. elegans lifespan. Mech Ageing Dev. 2002;123:765–771. - PubMed
-
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. - PubMed
-
- Gems D, McElwee JJ. Broad spectrum detoxification: the major longevity assurance process regulated by insulin/IGF-1 signaling? Mech Ageing Dev. 2005;126:381–387. - PubMed
-
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

