Localized fetomaternal hyperglycemia: spatial and kinetic definition by positron emission tomography
- PMID: 20700464
- PMCID: PMC2917372
- DOI: 10.1371/journal.pone.0012027
Localized fetomaternal hyperglycemia: spatial and kinetic definition by positron emission tomography
Abstract
Background: Complex but common maternal diseases such as diabetes and obesity contribute to adverse fetal outcomes. Understanding of the mechanisms involved is hampered by difficulty in isolating individual elements of complex maternal states in vivo. We approached this problem in the context of maternal diabetes and sought an approach to expose the developing fetus in vivo to isolated hyperglycemia in the pregnant rat.
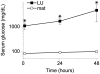
Methodology and principal findings: We hypothesized that glucose infused into the arterial supply of one uterine horn would more highly expose fetuses in the ipsilateral versus contralateral uterine horn. To test this, the glucose tracer [18F]fluorodeoxyglucose (FDG) was infused via the left uterine artery. Regional glucose uptake into maternal tissues and fetuses was quantified using positron emission tomography (PET). Upon infusion, FDG accumulation began in the left-sided placentae, subsequently spreading to the fetuses. Over two hours after completion of the infusion, FDG accumulation was significantly greater in left compared to right uterine horn fetuses, favoring the left by 1.9+/-0.1 and 2.8+/-0.3 fold under fasted and hyperinsulinemic conditions (p<10(-11) n=32-35 and p<10(-12) n=27-45) respectively. By contrast, centrally administered [3H]-2-deoxyglucose accumulated equally between the fetuses of the two uterine horns. Induction of significant hyperglycemia (10(3) mg/dL) localized to the left uterine artery was sustained for at least 48 hours while maternal euglycemia was maintained.
Conclusions and significance: This approach exposes selected fetuses to localized hyperglycemia in vivo, minimizing exposure of the mother and thus secondary effects. Additionally, a set of less exposed internal control fetuses are maintained for comparison, allowing direct study of the in vivo fetal effects of isolated hyperglycemia. Broadly, this approach can be extended to study a variety of maternal-sided perturbations suspected to directly affect fetal health.
Conflict of interest statement
Figures




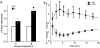
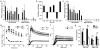
Similar articles
-
PET/CT imaging reveals unrivaled placental avidity for glucose compared to other tissues.Placenta. 2015 Feb;36(2):115-20. doi: 10.1016/j.placenta.2014.12.009. Epub 2014 Dec 23. Placenta. 2015. PMID: 25555498 Free PMC article.
-
Effect of insulin and dexamethasone on fetal assimilation of maternal glucose.Endocrinology. 2011 Jan;152(1):255-62. doi: 10.1210/en.2010-0959. Epub 2010 Nov 17. Endocrinology. 2011. PMID: 21084442 Free PMC article.
-
High glucose alters fetal rat islet transcriptome and induces progeny islet dysfunction.J Endocrinol. 2019 Feb 1;240(2):309-323. doi: 10.1530/JOE-18-0493. J Endocrinol. 2019. PMID: 30508415
-
Hyperglycemia and 18F-FDG PET/CT, issues and problem solving: a literature review.Acta Diabetol. 2020 Mar;57(3):253-262. doi: 10.1007/s00592-019-01385-8. Epub 2019 Jul 15. Acta Diabetol. 2020. PMID: 31304560 Review.
-
Maternal factors modulating nutrient transfer to fetus.Biol Neonate. 1987;51(2):86-93. doi: 10.1159/000242637. Biol Neonate. 1987. PMID: 3552062 Review.
Cited by
-
PET/CT imaging reveals unrivaled placental avidity for glucose compared to other tissues.Placenta. 2015 Feb;36(2):115-20. doi: 10.1016/j.placenta.2014.12.009. Epub 2014 Dec 23. Placenta. 2015. PMID: 25555498 Free PMC article.
-
Fetal hyperglycemia acutely induces persistent insulin resistance in skeletal muscle.J Endocrinol. 2019 Jul 1;242(1):M1-M15. doi: 10.1530/JOE-18-0455. J Endocrinol. 2019. PMID: 30444716 Free PMC article.
-
Effect of insulin and dexamethasone on fetal assimilation of maternal glucose.Endocrinology. 2011 Jan;152(1):255-62. doi: 10.1210/en.2010-0959. Epub 2010 Nov 17. Endocrinology. 2011. PMID: 21084442 Free PMC article.
-
Angiogenic imbalance and diminished matrix metalloproteinase-2 and -9 underlie regional decreases in uteroplacental vascularization and feto-placental growth in hypertensive pregnancy.Biochem Pharmacol. 2017 Dec 15;146:101-116. doi: 10.1016/j.bcp.2017.09.005. Epub 2017 Sep 11. Biochem Pharmacol. 2017. PMID: 28912068 Free PMC article.
-
The reduction in FOXA2 activity during lung development in fetuses from diabetic rat mothers is reversed by Akt inhibition.FEBS Open Bio. 2018 Sep 25;8(10):1594-1604. doi: 10.1002/2211-5463.12517. eCollection 2018 Oct. FEBS Open Bio. 2018. PMID: 30338211 Free PMC article.
References
-
- Finnell RH, Waes JG, Eudy JD, Rosenquist TH. Molecular basis of environmentally induced birth defects. Annu Rev Pharmacol Toxicol. 2002;42:181–208. - PubMed
-
- Maayan-Metzger A, Lubin D, Kuint J. Hypoglycemia rates in the first days of life among term infants born to diabetic mothers. Neonatology. 2009;96:80–85. - PubMed
-
- Weiss SJ, St Jonn-Seed M, Harris-Muchell C. The contribution of fetal drug exposure to temperament: potential teratogenic effects on neuropsychiatric risk. J Child Psychol Psychiatry. 2007;48:773–784. - PubMed
-
- Parman T, Wiley MJ, Wells PG. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat Med. 1999;5:582–585. - PubMed
-
- Daston GP, Yonker JE, Powers JF, Heitmeyer SA. Difference in teratogenic potency of ethylenethiourea in rats and mice: relative contribution of embryonic and maternal factors. Teratology. 1989;40:555–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

