Evidence for the existence of secretory granule (dense-core vesicle)-based inositol 1,4,5-trisphosphate-dependent Ca2+ signaling system in astrocytes
- PMID: 20700485
- PMCID: PMC2916839
- DOI: 10.1371/journal.pone.0011973
Evidence for the existence of secretory granule (dense-core vesicle)-based inositol 1,4,5-trisphosphate-dependent Ca2+ signaling system in astrocytes
Abstract
Background: The gliotransmitters released from astrocytes are deemed to play key roles in the glial cell-neuron communication for normal function of the brain. The gliotransmitters, such as glutamate, ATP, D-serine, neuropeptide Y, are stored in vesicles of astrocytes and secreted following the inositol 1,4,5-trisphosphate (IP3)-induced intracellular Ca2+ releases. Yet studies on the identity of the IP3-dependent intracellular Ca2+ stores remain virtually unexplored.
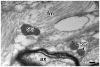
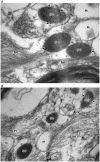
Principal findings: We have therefore studied the potential existence of the IP3-sensitive intracellular Ca2+ stores in the cytoplasm of astrocytes using human brain tissue samples in contrast to cultured astrocytes that had primarily been used in the past. It was thus found that secretory granule marker proteins chromogranins and secretogranin II localize in the large dense core vesicles of astrocytes, thereby confirming the large dense core vesicles as bona fide secretory granules. Moreover, consistent with the major IP3-dependent intracellular Ca2+ store role of secretory granules in secretory cells, secretory granules of astrocytes also contained all three (types 1, 2, and 3) IP3R isoforms.
Significance: Given that the secretory granule marker proteins chromogranins and secretogranin II are high-capacity, low-affinity Ca2+ storage proteins and chromogranins interact with the IP3Rs to activate the IP3R/Ca2+ channels, i.e., increase both the mean open time and the open probability of the channels, these results imply that secretory granules of astrocytes function as the IP3-sensitive intracellular Ca2+ store.
Conflict of interest statement
Figures





References
-
- Perea G, Araque A. Glial calcium signaling and neuron-glia communication. Cell Calcium. 2005;38:375–382. - PubMed
-
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. - PubMed
-
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. - PubMed
-
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54:700–715. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

