EPO receptor gain-of-function causes hereditary polycythemia, alters CD34 cell differentiation and increases circulating endothelial precursors
- PMID: 20700488
- PMCID: PMC2916842
- DOI: 10.1371/journal.pone.0012015
EPO receptor gain-of-function causes hereditary polycythemia, alters CD34 cell differentiation and increases circulating endothelial precursors
Retraction in
-
Retraction: EPO Receptor Gain-of-Function Causes Hereditary Polycythemia, Alters CD34+ Cell Differentiation and Increases Circulating Endothelial Precursors.PLoS One. 2020 Mar 4;15(3):e0230279. doi: 10.1371/journal.pone.0230279. eCollection 2020. PLoS One. 2020. PMID: 32130275 Free PMC article. No abstract available.
Abstract
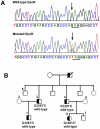
Background: Gain-of-function of erythropoietin receptor (EPOR) mutations represent the major cause of primary hereditary polycythemia. EPOR is also found in non-erythroid tissues, although its physiological role is still undefined.
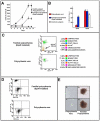
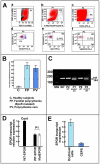
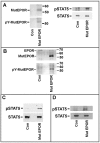
Methodology/principal findings: We describe a family with polycythemia due to a heterozygous mutation of the EPOR gene that causes a G-->T change at nucleotide 1251 of exon 8. The novel EPOR G1251T mutation results in the replacement of a glutamate residue by a stop codon at amino acid 393. Differently from polycythemia vera, EPOR G1251T CD34(+) cells proliferate and differentiate towards the erythroid phenotype in the presence of minimal amounts of EPO. Moreover, the affected individuals show a 20-fold increase of circulating endothelial precursors. The analysis of erythroid precursor membranes demonstrates a heretofore undescribed accumulation of the truncated EPOR, probably due to the absence of residues involved in the EPO-dependent receptor internalization and degradation. Mutated receptor expression in EPOR-negative cells results in EPOR and Stat5 phosphorylation. Moreover, patient erythroid precursors present an increased activation of EPOR and its effectors, including Stat5 and Erk1/2 pathway.
Conclusions/significance: Our data provide an unanticipated mechanism for autosomal dominant inherited polycythemia due to a heterozygous EPOR mutation and suggest a regulatory role of EPO/EPOR pathway in human circulating endothelial precursors homeostasis.
Conflict of interest statement
Figures


Similar articles
-
Dynamic ligand modulation of EPO receptor pools, and dysregulation by polycythemia-associated EPOR alleles.PLoS One. 2012;7(1):e29064. doi: 10.1371/journal.pone.0029064. Epub 2012 Jan 12. PLoS One. 2012. PMID: 22253704 Free PMC article.
-
Erythropoietin hypersensitivity in primary familial and congenital polycythemia: role of tyrosines Y285 and Y344 in erythropoietin receptor cytoplasmic domain.Biochim Biophys Acta. 2005 Apr 15;1740(1):17-28. doi: 10.1016/j.bbadis.2005.03.003. Epub 2005 Apr 1. Biochim Biophys Acta. 2005. PMID: 15878737
-
Delayed hemoglobin switching and perinatal neocytolysis in mice with gain-of-function erythropoietin receptor.J Mol Med (Berl). 2016 May;94(5):597-608. doi: 10.1007/s00109-015-1375-y. Epub 2015 Dec 26. J Mol Med (Berl). 2016. PMID: 26706855 Free PMC article.
-
Polycythemia and oxygen sensing.Pathol Biol (Paris). 2004 Jun;52(5):280-4. doi: 10.1016/j.patbio.2004.02.006. Pathol Biol (Paris). 2004. PMID: 15217714 Review.
-
Genetic variants of erythropoietin (EPO) and EPO receptor genes in familial erythrocytosis.Int J Lab Hematol. 2019 Apr;41(2):162-167. doi: 10.1111/ijlh.12949. Epub 2018 Dec 3. Int J Lab Hematol. 2019. PMID: 30507031 Free PMC article. Review.
Cited by
-
Dynamic ligand modulation of EPO receptor pools, and dysregulation by polycythemia-associated EPOR alleles.PLoS One. 2012;7(1):e29064. doi: 10.1371/journal.pone.0029064. Epub 2012 Jan 12. PLoS One. 2012. PMID: 22253704 Free PMC article.
-
JAK2 unmutated erythrocytosis: current diagnostic approach and therapeutic views.Leukemia. 2021 Aug;35(8):2166-2181. doi: 10.1038/s41375-021-01290-6. Epub 2021 May 21. Leukemia. 2021. PMID: 34021251 Free PMC article. Review.
-
Erythropoietin: A Personal Alice in Wonderland Trip in the Shadow of the Giants.Biomolecules. 2024 Mar 27;14(4):408. doi: 10.3390/biom14040408. Biomolecules. 2024. PMID: 38672425 Free PMC article. Review.
-
Retraction: EPO Receptor Gain-of-Function Causes Hereditary Polycythemia, Alters CD34+ Cell Differentiation and Increases Circulating Endothelial Precursors.PLoS One. 2020 Mar 4;15(3):e0230279. doi: 10.1371/journal.pone.0230279. eCollection 2020. PLoS One. 2020. PMID: 32130275 Free PMC article. No abstract available.
-
Myeloproliferative neoplasms and the JAK/STAT signaling pathway: an overview.Rev Bras Hematol Hemoter. 2015 Sep-Oct;37(5):348-53. doi: 10.1016/j.bjhh.2014.10.001. Epub 2015 Jun 9. Rev Bras Hematol Hemoter. 2015. PMID: 26408371 Free PMC article.
References
-
- Barber DL, D'Andrea AD. The erythropoietin receptor and the molecular basis of signal transduction. Semin Hematol. 1992;29:293–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

