The insular taste cortex contributes to odor quality coding
- PMID: 20700500
- PMCID: PMC2917218
- DOI: 10.3389/fnhum.2010.00058
The insular taste cortex contributes to odor quality coding
Abstract
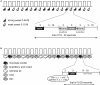
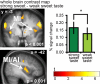
Despite distinct peripheral and central pathways, stimulation of both the olfactory and the gustatory systems may give rise to the sensation of sweetness. Whether there is a common central mechanism producing sweet quality sensations or two discrete mechanisms associated independently with gustatory and olfactory stimuli is currently unknown. Here we used fMRI to determine whether odor sweetness is represented in the piriform olfactory cortex, which is thought to code odor quality, or in the insular taste cortex, which is thought to code taste quality. Fifteen participants sampled two concentrations of a pure sweet taste (sucrose), two sweet food odors (chocolate and strawberry), and two sweet floral odors (lilac and rose). Replicating prior work we found that olfactory stimulation activated the piriform, orbitofrontal and insular cortices. Of these regions, only the insula also responded to sweet taste. More importantly, the magnitude of the response to the food odors, but not to the non-food odors, in this region of insula was positively correlated with odor sweetness rating. These findings demonstrate that insular taste cortex contributes to odor quality coding by representing the taste-like aspects of food odors. Since the effect was specific to the food odors, and only food odors are experienced with taste, we suggest this common central mechanism develops as a function of experiencing flavors.
Keywords: fMRI; flavor; gustatory; insula; multimodal; olfactory; piriform; sweet.
Figures


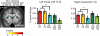

References
Grants and funding
LinkOut - more resources
Full Text Sources

