NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs
- PMID: 20700504
- PMCID: PMC2917352
- DOI: 10.1371/journal.pone.0011966
NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs
Abstract
Background: Terminal differentiation of NK cells is crucial in maintaining broad responsiveness to pathogens and discriminating normal cells from cells in distress. Although it is well established that KIRs, in conjunction with NKG2A, play a major role in the NK cell education that determines whether cells will end up competent or hyporesponsive, the events underlying the differentiation are still debated.
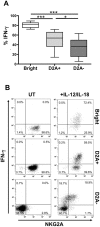
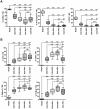
Methodology/principal findings: A combination of complementary approaches to assess the kinetics of the appearance of each subset during development allowed us to obtain new insights into these terminal stages of differentiation, characterising their gene expression profiles at a pan-genomic level, their distinct surface receptor patterns and their prototypic effector functions. The present study supports the hypothesis that CD56dim cells derive from the CD56bright subset and suggests that NK cell responsiveness is determined by persistent inhibitory signals received during their education. We report here the inverse correlation of NKG2A expression with KIR expression and explore whether this correlation bestows functional competence on NK cells. We show that CD56dimNKG2A-KIR+ cells display the most differentiated phenotype associated to their unique ability to respond against HLA-E+ target cells. Importantly, after IL-12+IL-18 stimulation, reacquisition of NKG2A strongly correlates with IFN-gamma production in CD56dimNKG2A- NK cells.
Conclusions/significance: Together, these findings call for the reclassification of mature human NK cells into distinct subsets and support a new model, in which the NK cell differentiation and functional fate are based on a stepwise decrease of NKG2A and acquisition of KIRs.
Conflict of interest statement
Figures





References
-
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. - PubMed
-
- Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 2008;15:226–233. - PubMed
-
- Lanier LL. Natural killer cell receptor signaling. Curr Opin Immunol. 2003;15:308–314. - PubMed
-
- Ouyang Q, Baerlocher G, Vulto I, Lansdorp PM. Telomere length in human natural killer cell subsets. Ann N Y Acad Sci. 2007;1106:240–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

