Dual beneficial effects of (-)-epigallocatechin-3-gallate on levodopa methylation and hippocampal neurodegeneration: in vitro and in vivo studies
- PMID: 20700524
- PMCID: PMC2916818
- DOI: 10.1371/journal.pone.0011951
Dual beneficial effects of (-)-epigallocatechin-3-gallate on levodopa methylation and hippocampal neurodegeneration: in vitro and in vivo studies
Abstract
Background: A combination of levodopa (L-DOPA) and carbidopa is the most commonly-used treatment for symptom management in Parkinson's disease. Studies have shown that concomitant use of a COMT inhibitor is highly beneficial in controlling the wearing-off phenomenon by improving L-DOPA bioavailability as well as brain entry. The present study sought to determine whether (-)-epigallocatechin-3-gallate (EGCG), a common tea polyphenol, can serve as a naturally-occurring COMT inhibitor that also possesses neuroprotective actions.
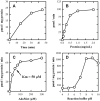
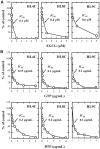
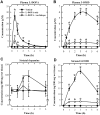
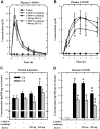
Methodology/principal findings: Using both in vitro and in vivo models, we investigated the modulating effects of EGCG on L-DOPA methylation as well as on chemically induced oxidative neuronal damage and degeneration. EGCG strongly inhibited human liver COMT-mediated O-methylation of L-DOPA in a concentration-dependent manner in vitro, with an average IC50 of 0.36 microM. Oral administration of EGCG moderately lowered the accumulation of 3-O-methyldopa in the plasma and striatum of rats treated with L-DOPA+carbidopa. In addition, EGCG also reduced glutamate-induced oxidative cytotoxicity in cultured HT22 mouse hippocampal neuronal cells through inactivation of the nuclear factor kappaB-signaling pathway. Under in vivo conditions, administration of EGCG exerted a strong protective effect against kainic acid-induced oxidative neuronal death in the hippocampus of rats.
Conclusions/significance: These observations suggest that oral administration of EGCG may have significant beneficial effects in Parkinson's patients treated with L-DOPA and carbidopa by exerting a modest inhibition of L-DOPA methylation plus a strong neuroprotection against oxidative damage and degeneration.
Conflict of interest statement
Figures


Similar articles
-
Beneficial effects of natural phenolics on levodopa methylation and oxidative neurodegeneration.Brain Res. 2013 Feb 25;1497:1-14. doi: 10.1016/j.brainres.2012.11.043. Epub 2012 Dec 1. Brain Res. 2013. PMID: 23206800 Free PMC article.
-
Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (-)-epigallocatechin gallate.Drug Metab Dispos. 2003 May;31(5):572-9. doi: 10.1124/dmd.31.5.572. Drug Metab Dispos. 2003. PMID: 12695345
-
Bioactive components and mechanisms of Pu-erh tea in improving levodopa metabolism in rats through COMT inhibition.Food Funct. 2024 May 20;15(10):5287-5299. doi: 10.1039/d4fo00538d. Food Funct. 2024. PMID: 38639730
-
Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson's disease.Drugs. 2000 Jun;59(6):1233-50. doi: 10.2165/00003495-200059060-00004. Drugs. 2000. PMID: 10882160 Review.
-
Pharmacokinetics and pharmacodynamics of levodopa/carbidopa cotherapies for Parkinson's disease.Expert Opin Drug Metab Toxicol. 2020 May;16(5):403-414. doi: 10.1080/17425255.2020.1750596. Epub 2020 Apr 27. Expert Opin Drug Metab Toxicol. 2020. PMID: 32238065 Review.
Cited by
-
Neurodegenerative Etiology of Aromatic L-Amino Acid Decarboxylase Deficiency: a Novel Concept for Expanding Treatment Strategies.Mol Neurobiol. 2024 May;61(5):2996-3018. doi: 10.1007/s12035-023-03684-2. Epub 2023 Nov 13. Mol Neurobiol. 2024. PMID: 37953352 Review.
-
Targeting natural antioxidant polyphenols to protect neuroinflammation and neurodegenerative diseases: a comprehensive review.Front Pharmacol. 2025 Jan 24;16:1492517. doi: 10.3389/fphar.2025.1492517. eCollection 2025. Front Pharmacol. 2025. PMID: 39981183 Free PMC article. Review.
-
Neuroprotective Polyphenols: A Modulatory Action on Neurotransmitter Pathways.Curr Neuropharmacol. 2020;18(5):431-445. doi: 10.2174/1570159X18666200106155127. Curr Neuropharmacol. 2020. PMID: 31903883 Free PMC article. Review.
-
Considerations for the Use of Polyphenols as Therapies in Neurodegenerative Diseases.Int J Mol Sci. 2019 Apr 16;20(8):1883. doi: 10.3390/ijms20081883. Int J Mol Sci. 2019. PMID: 30995776 Free PMC article. Review.
-
Reduction in Autophagy by (-)-Epigallocatechin-3-Gallate (EGCG): a Potential Mechanism of Prevention of Mitochondrial Dysfunction After Subarachnoid Hemorrhage.Mol Neurobiol. 2017 Jan;54(1):392-405. doi: 10.1007/s12035-015-9629-9. Epub 2016 Jan 7. Mol Neurobiol. 2017. PMID: 26742518
References
-
- Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann NY Acad Sci. 2003;991:1–14. - PubMed
-
- Toulouse A, Sullivan AM. Progress in Parkinson's disease-Where do we stand? Prog Neurobiol. 2008;85:376–392. - PubMed
-
- Morgan JC, Sethi KD. Emerging drugs for Parkinson's disease. Expert Opin Emer Drugs. 2006;11:403–417. - PubMed
-
- Schapira AH, Obeso JA, Olanow CW. The place of COMT inhibitors in the armamentarium of drugs for the treatment of Parkinson's disease. Neurology. 2000;55:S65–S68. - PubMed
-
- Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

