Calcineurin interacts with PERK and dephosphorylates calnexin to relieve ER stress in mammals and frogs
- PMID: 20700529
- PMCID: PMC2916823
- DOI: 10.1371/journal.pone.0011925
Calcineurin interacts with PERK and dephosphorylates calnexin to relieve ER stress in mammals and frogs
Abstract
Background: The accumulation of misfolded proteins within the endoplasmic reticulum (ER) triggers a cellular process known as the Unfolded Protein Response (UPR). One of the earliest responses is the attenuation of protein translation. Little is known about the role that Ca2+ mobilization plays in the early UPR. Work from our group has shown that cytosolic phosphorylation of calnexin (CLNX) controls Ca2+ uptake into the ER via the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) 2b.
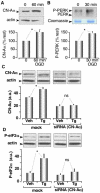
Methodology/principal findings: Here, we demonstrate that calcineurin (CN), a Ca2+ dependent phosphatase, associates with the (PKR)-like ER kinase (PERK), and promotes PERK auto-phosphorylation. This association, in turn, increases the phosphorylation level of eukaryotic initiation factor-2 alpha (eIF2-alpha) and attenuates protein translation. Data supporting these conclusions were obtained from co-immunoprecipitations, pull-down assays, in-vitro kinase assays, siRNA treatments and [35S]-methionine incorporation measurements. The interaction of CN with PERK was facilitated at elevated cytosolic Ca2+ concentrations and involved the cytosolic domain of PERK. CN levels were rapidly increased by ER stressors, which could be blocked by siRNA treatments for CN-Aalpha in cultured astrocytes. Downregulation of CN blocked subsequent ER-stress-induced increases in phosphorylated elF2-alpha. CN knockdown in Xenopus oocytes predisposed them to induction of apoptosis. We also found that CLNX was dephosphorylated by CN when Ca2+ increased. These data were obtained from [gamma32P]-CLNX immunoprecipitations and Ca2+ imaging measurements. CLNX was dephosphorylated when Xenopus oocytes were treated with ER stressors. Dephosphorylation was pharmacologically blocked by treatment with CN inhibitors. Finally, evidence is presented that PERK phosphorylates CN-A at low resting levels of Ca2+. We further show that phosphorylated CN-A exhibits decreased phosphatase activity, consistent with this regulatory mechanism being shut down as ER homeostasis is re-established.
Conclusions/significance: Our data suggest two new complementary roles for CN in the regulation of the early UPR. First, CN binding to PERK enhances inhibition of protein translation to allow the cell time to recover. The induction of the early UPR, as indicated by increased P-elF2alpha, is critically dependent on a translational increase in CN-Aalpha. Second, CN dephosphorylates CLNX and likely removes inhibition of SERCA2b activity, which would aid the rapid restoration of ER Ca2+ homeostasis.
Conflict of interest statement
Figures


Similar articles
-
Cytosolic phosphorylation of calnexin controls intracellular Ca(2+) oscillations via an interaction with SERCA2b.J Cell Biol. 2000 Jun 12;149(6):1235-48. doi: 10.1083/jcb.149.6.1235. J Cell Biol. 2000. PMID: 10851021 Free PMC article.
-
The Human Cytomegalovirus Endoplasmic Reticulum-Resident Glycoprotein UL148 Activates the Unfolded Protein Response.J Virol. 2018 Sep 26;92(20):e00896-18. doi: 10.1128/JVI.00896-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30045994 Free PMC article.
-
Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2.J Biol Chem. 2011 Feb 11;286(6):4809-18. doi: 10.1074/jbc.M110.152900. Epub 2010 Dec 6. J Biol Chem. 2011. PMID: 21135100 Free PMC article.
-
Endoplasmic reticulum stress and eIF2α phosphorylation: The Achilles heel of pancreatic β cells.Mol Metab. 2017 Jul 12;6(9):1024-1039. doi: 10.1016/j.molmet.2017.06.001. eCollection 2017 Sep. Mol Metab. 2017. PMID: 28951826 Free PMC article. Review.
-
Translational control and the unfolded protein response.Antioxid Redox Signal. 2007 Dec;9(12):2357-71. doi: 10.1089/ars.2007.1764. Antioxid Redox Signal. 2007. PMID: 17760508 Review.
Cited by
-
CRELD2 is a novel modulator of calcium release and calcineurin-NFAT signalling during osteoclast differentiation.Sci Rep. 2022 Aug 16;12(1):13884. doi: 10.1038/s41598-022-17347-0. Sci Rep. 2022. PMID: 35974042 Free PMC article.
-
Stanniocalcin 2 alters PERK signalling and reduces cellular injury during cerulein induced pancreatitis in mice.BMC Cell Biol. 2011 May 5;12:17. doi: 10.1186/1471-2121-12-17. BMC Cell Biol. 2011. PMID: 21545732 Free PMC article.
-
The Prenylflavonoid Xanthohumol Reduces Alzheimer-Like Changes and Modulates Multiple Pathogenic Molecular Pathways in the Neuro2a/APPswe Cell Model of AD.Front Pharmacol. 2018 Apr 4;9:199. doi: 10.3389/fphar.2018.00199. eCollection 2018. Front Pharmacol. 2018. PMID: 29670521 Free PMC article.
-
Calcium Contributes to the Cytotoxic Interaction Between Diclofenac and Cytokines.Toxicol Sci. 2016 Feb;149(2):372-84. doi: 10.1093/toxsci/kfv249. Epub 2015 Nov 24. Toxicol Sci. 2016. PMID: 26609140 Free PMC article.
-
6-Shogaol induces apoptosis in human hepatocellular carcinoma cells and exhibits anti-tumor activity in vivo through endoplasmic reticulum stress.PLoS One. 2012;7(6):e39664. doi: 10.1371/journal.pone.0039664. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768104 Free PMC article.
References
-
- Trombetta SE, Parodi AJ. Purification to apparent homogeneity and partial characterization of rat liver UDP-glucose:glycoprotein glucosyltransferase. J Biol Chem. 1992;267:9236–9240. - PubMed
-
- Lodish HF, Kong N, Wikstrom L. Calcium is required for folding of newly made subunits of the asialoglycoprotein receptor within the endoplasmic reticulum. J Biol Chem. 1992;267:12753–12760. - PubMed
-
- Brostrom MA, Brostrom CO. Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: implications for cell growth and adaptability. Cell Calcium. 2003;34:345–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

