Oxytocin signaling in mouse taste buds
- PMID: 20700536
- PMCID: PMC2916830
- DOI: 10.1371/journal.pone.0011980
Oxytocin signaling in mouse taste buds
Abstract
Background: The neuropeptide, oxytocin (OXT), acts on brain circuits to inhibit food intake. Mutant mice lacking OXT (OXT knockout) overconsume salty and sweet (i.e. sucrose, saccharin) solutions. We asked if OXT might also act on taste buds via its receptor, OXTR.
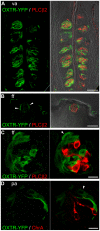
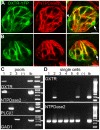
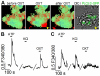
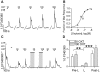
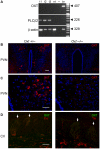
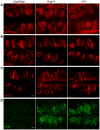
Methodology/principal findings: Using RT-PCR, we detected the expression of OXTR in taste buds throughout the oral cavity, but not in adjacent non-taste lingual epithelium. By immunostaining tissues from OXTR-YFP knock-in mice, we found that OXTR is expressed in a subset of Glial-like (Type I) taste cells, and also in cells on the periphery of taste buds. Single-cell RT-PCR confirmed this cell-type assignment. Using Ca2+ imaging, we observed that physiologically appropriate concentrations of OXT evoked [Ca2+]i mobilization in a subset of taste cells (EC50 approximately 33 nM). OXT-evoked responses were significantly inhibited by the OXTR antagonist, L-371,257. Isolated OXT-responsive taste cells were neither Receptor (Type II) nor Presynaptic (Type III) cells, consistent with our immunofluorescence observations. We also investigated the source of OXT peptide that may act on taste cells. Both RT-PCR and immunostaining suggest that the OXT peptide is not produced in taste buds or in their associated nerves. Finally, we also examined the morphology of taste buds from mice that lack OXTR. Taste buds and their constituent cell types appeared very similar in mice with two, one or no copies of the OXTR gene.
Conclusions/significance: We conclude that OXT elicits Ca2+ signals via OXTR in murine taste buds. OXT-responsive cells are most likely a subset of Glial-like (Type I) taste cells. OXT itself is not produced locally in taste tissue and is likely delivered through the circulation. Loss of OXTR does not grossly alter the morphology of any of the cell types contained in taste buds. Instead, we speculate that OXT-responsive Glial-like (Type I) taste bud cells modulate taste signaling and afferent sensory output. Such modulation would complement central pathways of appetite regulation that employ circulating homeostatic and satiety signals.
Conflict of interest statement
Figures

Similar articles
-
Cell proliferation and anti-oxidant effects of oxytocin and oxytocin receptors: role of extracellular signal-regulating kinase in astrocyte-like cells.Endocr Regul. 2020 Jul 1;54(3):172-182. doi: 10.2478/enr-2020-0020. Endocr Regul. 2020. PMID: 32857718
-
Oxytocin receptor gene loss influences expression of the oxytocin gene in C57BL/6J mice in a sex- and age-dependent manner.J Neuroendocrinol. 2020 Feb;32(2):e12821. doi: 10.1111/jne.12821. Epub 2020 Feb 11. J Neuroendocrinol. 2020. PMID: 31845417 Free PMC article.
-
Whole-Brain Wiring Diagram of Oxytocin System in Adult Mice.J Neurosci. 2022 Jun 22;42(25):5021-5033. doi: 10.1523/JNEUROSCI.0307-22.2022. Epub 2022 May 23. J Neurosci. 2022. PMID: 35606144 Free PMC article.
-
The Oxytocin Receptor: From Intracellular Signaling to Behavior.Physiol Rev. 2018 Jul 1;98(3):1805-1908. doi: 10.1152/physrev.00031.2017. Physiol Rev. 2018. PMID: 29897293 Review.
-
The New Frontier in Oxytocin Physiology: The Oxytonic Contraction.Int J Mol Sci. 2020 Jul 21;21(14):5144. doi: 10.3390/ijms21145144. Int J Mol Sci. 2020. PMID: 32708109 Free PMC article. Review.
Cited by
-
CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes.Nature. 2013 Mar 14;495(7440):223-6. doi: 10.1038/nature11906. Epub 2013 Mar 6. Nature. 2013. PMID: 23467090 Free PMC article.
-
GAD65Cre Drives Reporter Expression in Multiple Taste Cell Types.Chem Senses. 2021 Jan 1;46:bjab033. doi: 10.1093/chemse/bjab033. Chem Senses. 2021. PMID: 34160573 Free PMC article.
-
Genetics of mouse behavioral and peripheral neural responses to sucrose.Mamm Genome. 2021 Apr;32(2):51-69. doi: 10.1007/s00335-021-09858-4. Epub 2021 Mar 13. Mamm Genome. 2021. PMID: 33713179
-
Functional expression of TMEM16A in taste bud cells.J Physiol. 2021 Aug;599(15):3697-3714. doi: 10.1113/JP281645. Epub 2021 Jun 28. J Physiol. 2021. PMID: 34089532 Free PMC article.
-
Neural Functions of Hypothalamic Oxytocin and its Regulation.ASN Neuro. 2022 Jan-Dec;14:17590914221100706. doi: 10.1177/17590914221100706. ASN Neuro. 2022. PMID: 35593066 Free PMC article. Review.
References
-
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. - PubMed
-
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. - PubMed
-
- Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–R96. - PubMed
-
- Sabatier N, Rowe I, Leng G. Central release of oxytocin and the ventromedial hypothalamus. Biochem Soc Trans. 2007;35:1247–1251. - PubMed
-
- Arletti R, Benelli A, Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiol Behav. 1990;48:825–830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

