Candida albicans AGE3, the ortholog of the S. cerevisiae ARF-GAP-encoding gene GCS1, is required for hyphal growth and drug resistance
- PMID: 20700541
- PMCID: PMC2916835
- DOI: 10.1371/journal.pone.0011993
Candida albicans AGE3, the ortholog of the S. cerevisiae ARF-GAP-encoding gene GCS1, is required for hyphal growth and drug resistance
Abstract
Background: Hyphal growth and multidrug resistance of C. albicans are important features for virulence and antifungal therapy of this pathogenic fungus.
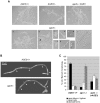
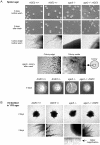
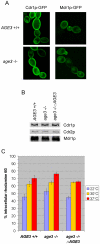
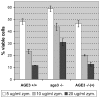
Methodology/principal findings: Here we show by phenotypic complementation analysis that the C. albicans gene AGE3 is the functional ortholog of the yeast ARF-GAP-encoding gene GCS1. The finding that the gene is required for efficient endocytosis points to an important functional role of Age3p in endosomal compartments. Most C. albicans age3Delta mutant cells which grew as cell clusters under yeast growth conditions showed defects in filamentation under different hyphal growth conditions and were almost completely disabled for invasive filamentous growth. Under hyphal growth conditions only a fraction of age3Delta cells shows a wild-type-like polarization pattern of the actin cytoskeleton and lipid rafts. Moreover, age3Delta cells were highly susceptible to several unrelated toxic compounds including antifungal azole drugs. Irrespective of the AGE3 genotype, C-terminal fusions of GFP to the drug efflux pumps Cdr1p and Mdr1p were predominantly localized in the plasma membrane. Moreover, the plasma membranes of wild-type and age3Delta mutant cells contained similar amounts of Cdr1p, Cdr2p and Mdr1p.
Conclusions/significance: The results indicate that the defect in sustaining filament elongation is probably caused by the failure of age3Delta cells to polarize the actin cytoskeleton and possibly of inefficient endocytosis. The high susceptibility of age3Delta cells to azoles is not caused by inefficient transport of efflux pumps to the cell membrane. A possible role of a vacuolar defect of age3Delta cells in drug susceptibility is proposed and discussed. In conclusion, our study shows that the ARF-GAP Age3p is required for hyphal growth which is an important virulence factor of C. albicans and essential for detoxification of azole drugs which are routinely used for antifungal therapy. Thus, it represents a promising antifungal drug target.
Conflict of interest statement
Figures
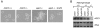



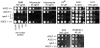
Similar articles
-
The lipid flippase subunit Cdc50 is required for antifungal drug resistance, endocytosis, hyphal development and virulence in Candida albicans.FEMS Yeast Res. 2019 May 1;19(3):foz033. doi: 10.1093/femsyr/foz033. FEMS Yeast Res. 2019. PMID: 31004489
-
Rsr1 focuses Cdc42 activity at hyphal tips and promotes maintenance of hyphal development in Candida albicans.Eukaryot Cell. 2013 Apr;12(4):482-95. doi: 10.1128/EC.00294-12. Epub 2012 Dec 7. Eukaryot Cell. 2013. PMID: 23223038 Free PMC article.
-
ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates.Antimicrob Agents Chemother. 2008 Nov;52(11):3851-62. doi: 10.1128/AAC.00463-08. Epub 2008 Aug 18. Antimicrob Agents Chemother. 2008. PMID: 18710914 Free PMC article.
-
Candida albicans hyphal initiation and elongation.Trends Microbiol. 2014 Dec;22(12):707-14. doi: 10.1016/j.tim.2014.09.001. Epub 2014 Sep 25. Trends Microbiol. 2014. PMID: 25262420 Free PMC article. Review.
-
Messenger RNA transport in the opportunistic fungal pathogen Candida albicans.Curr Genet. 2017 Dec;63(6):989-995. doi: 10.1007/s00294-017-0707-6. Epub 2017 May 16. Curr Genet. 2017. PMID: 28512683 Free PMC article. Review.
Cited by
-
Candida albicans ENT2 Contributes to Efficient Endocytosis, Cell Wall Integrity, Filamentation, and Virulence.mSphere. 2021 Oct 27;6(5):e0070721. doi: 10.1128/mSphere.00707-21. Epub 2021 Sep 29. mSphere. 2021. PMID: 34585966 Free PMC article.
-
The Genomes of Three Uneven Siblings: Footprints of the Lifestyles of Three Trichoderma Species.Microbiol Mol Biol Rev. 2016 Feb 10;80(1):205-327. doi: 10.1128/MMBR.00040-15. Print 2016 Mar. Microbiol Mol Biol Rev. 2016. PMID: 26864432 Free PMC article. Review.
-
The Role of Secretory Pathways in Candida albicans Pathogenesis.J Fungi (Basel). 2020 Feb 24;6(1):26. doi: 10.3390/jof6010026. J Fungi (Basel). 2020. PMID: 32102426 Free PMC article. Review.
-
Characterization of the chromosome 4 genes that affect fluconazole-induced disomy formation in Cryptococcus neoformans.PLoS One. 2012;7(3):e33022. doi: 10.1371/journal.pone.0033022. Epub 2012 Mar 7. PLoS One. 2012. PMID: 22412978 Free PMC article.
-
Slow Growth and Increased Spontaneous Mutation Frequency in Respiratory Deficient afo1- Yeast Suppressed by a Dominant Mutation in ATP3.G3 (Bethesda). 2020 Dec 3;10(12):4637-4648. doi: 10.1534/g3.120.401537. G3 (Bethesda). 2020. PMID: 33093184 Free PMC article.
References
-
- Soll DR, Morrow B, Srikantha T. High-frequency phenotypic switching in Candida albicans. Trends Genet. 1993;9:61–65. - PubMed
-
- Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004;12:317–324. - PubMed
-
- Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–335. - PubMed
-
- Kumamoto CA, Vinces MD. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell Microbiol. 2005;7:1546–1554. - PubMed
-
- Ernst JF. Transcription factors in Candida albicans - environmental control of morphogenesis. Microbiology. 2000;146(Pt 8):1763–1774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

